
The PLA2022 Europe is back at the Lake Maggiore for a live edition.
It is an opportunity for visitors and providers to re-connect with human interactions, after so many video-calls, webinars and virtual events.
We are building a great program for great discussions. Scroll down and discover the Topics and the Speakers
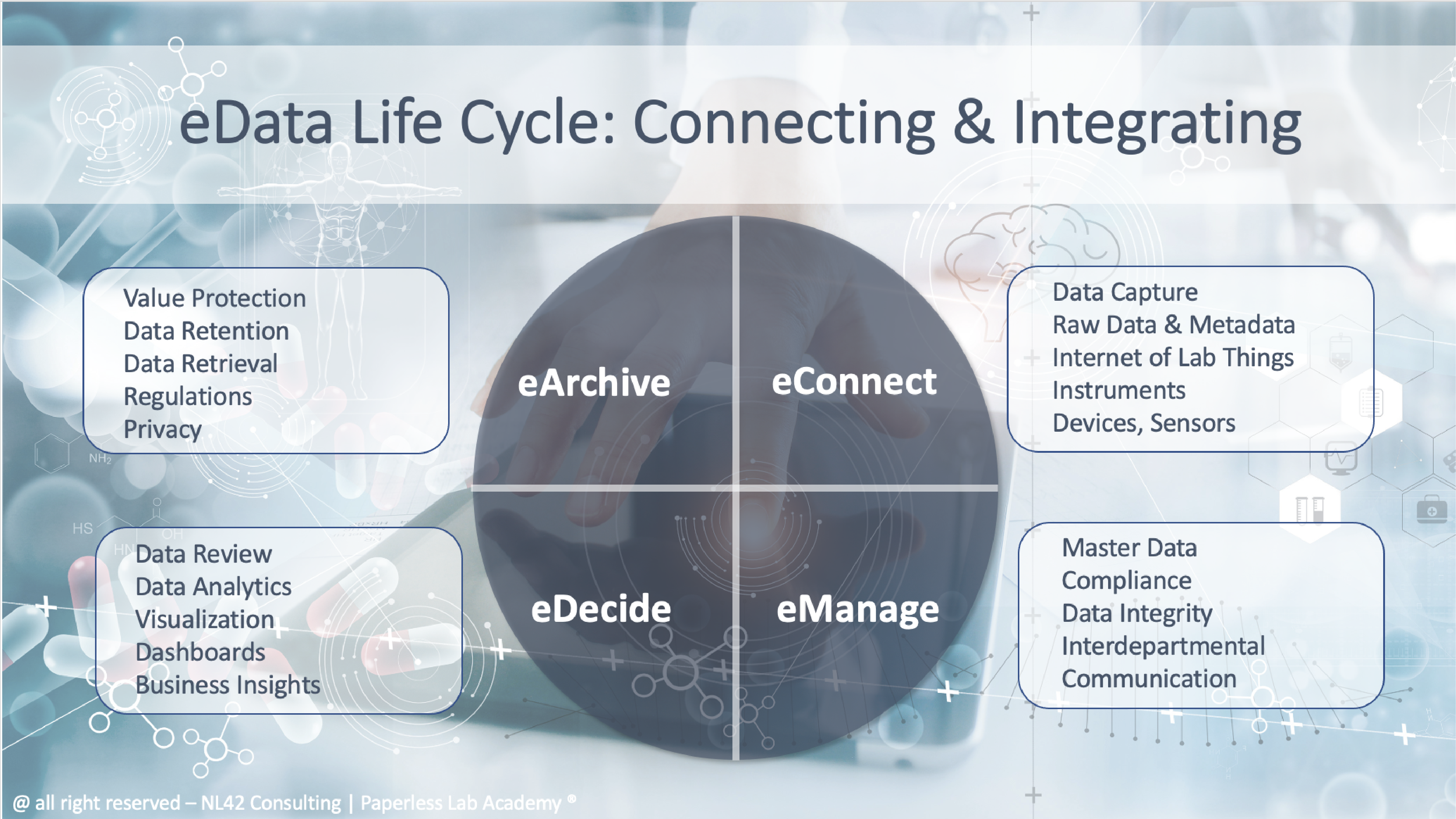
eData Lifecycle Discussions
Digital data have a specific lifecycle from their creation to final storing. At every step those data should be easily reachable and bring the necessary information to facilitate the decision making. Industry compliances also matter here and need to be covered. Yet above all the right management of those data is crucial for its company business.
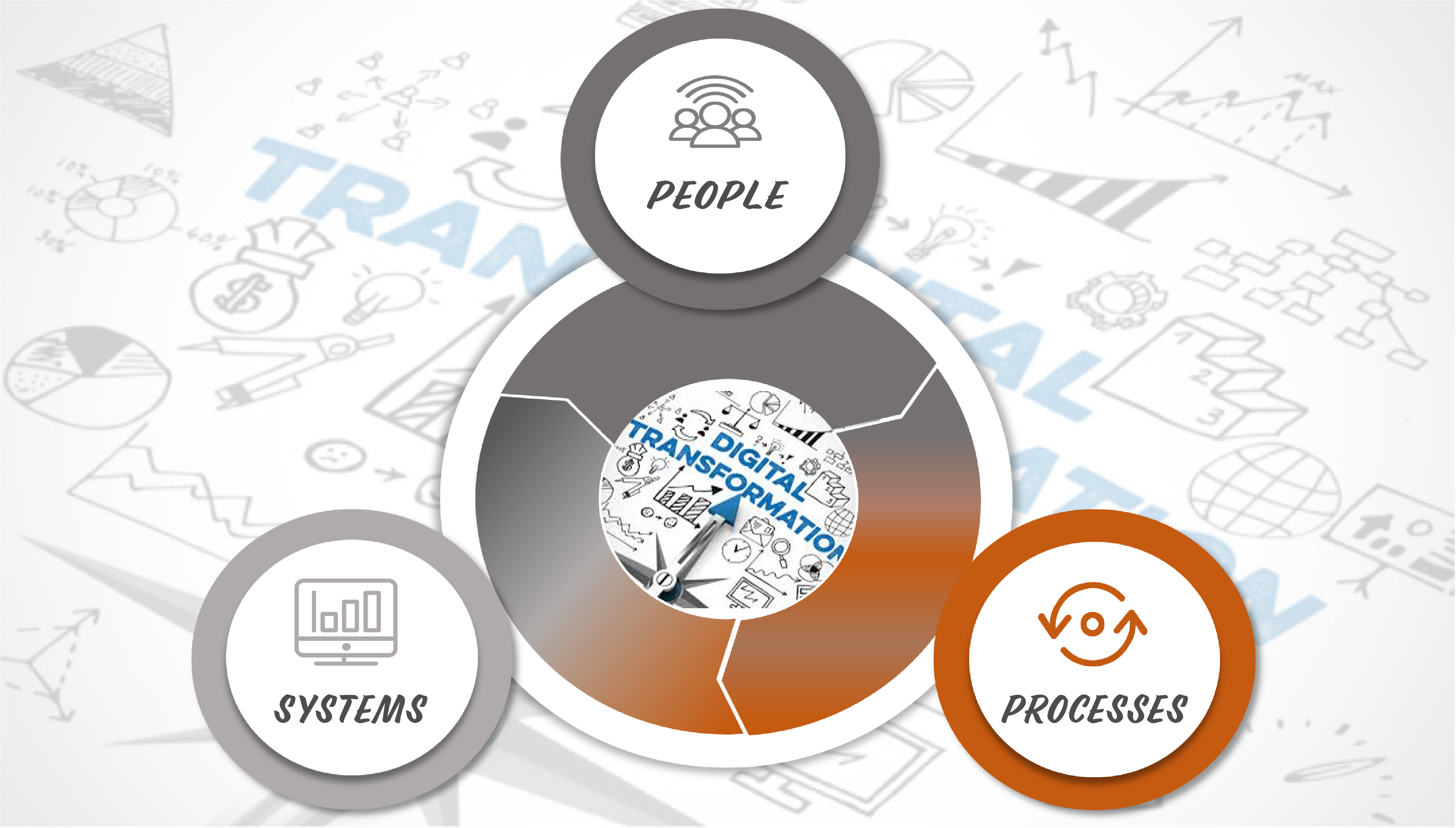
Driving the Change Management
Managing a Digital Transformation project in your company for your laboratory and quality processes is not a flawless project. Beside Your processes and the adequate systems to be selected and implemented, every one that have been through it will always reinforce the point that getting your team on board is crucial. The Human Factor is key for a successful digital transformation project.

Digital Data Management Topics
Topics covered are about Digital Data Management all along the eData lifecycle and all the related matters such as data integrity, data integration, data analytics, data standardisation, structured vs unstructured data, data search, machine learning, artificial intelligence, etc.
PLA2022 Europe Program

 Isabel Muñoz-Willery Ph.D. & Roberto Castelnovo, owners & organizers at NL42 Consulting | Paperless Lab Academy®
Isabel Muñoz-Willery Ph.D. & Roberto Castelnovo, owners & organizers at NL42 Consulting | Paperless Lab Academy®
Isabel and Roberto are entrepreneurial persons involved in paperless laboratory processes projects. Coming from international managerial roles with strong domain knowledge in laboratory processes, laboratory automation, laboratory informatics, and project management, they launched their personal project, NL42 consulting, by 2012. Soon their own customers were asking for better visibility of the industry solutions and learn about the latest technological trends and best-recommended methodologies.
Along the time, the Paperless Lab Academy® has become the platform where to find the answers.

Andreas Steinle, Manager Digital Solution at Roche Diagnostics GmbH
#Cloud Strategy #Leasson Learned #FAIR #Agile
Andreas Steinle has been developing and driving the digital transformation strategy for Roche Pharmaceuticals Technical Development Europe Europe since 2018.
He is a Product Owner accountable for building a cloud-based platform for instrument and device integration with FAIR data principles and works as a coach for the implementation of global solutions such as Benchling ELN /Labware LIMS.
Before 2018, Andreas was responsible for the support and validation of analytical systems and laboratory equipment (pH meters, HPLC, LIMS, archiving, etc.) at Roche Pharma Biotech Production in Penzberg as head of the "Analytical Systems" group.
 Discover why Roche's PTD cloud strategy enables faster access to new treatments for patients.
Discover why Roche's PTD cloud strategy enables faster access to new treatments for patients.
You can only learn things by trying them out!
See through lessons learned examples (e.g., Benchling implementation, data collection, and business-oriented use cases) , how FAIR data, digital transformation, and an agile mindset combined with a cloud strategy became reality over time.
The mix of our vision, strategy, as-is state, lessons learned and the validation strategy will provide you with meaningful insights for your own cloud strategy.
 Simon Brem, Head Laboratory Systems & Standards, CGAM LIMS Project Lead at Carbogen Amcis
Simon Brem, Head Laboratory Systems & Standards, CGAM LIMS Project Lead at Carbogen Amcis
#Paperless Initiatives #Business Process Design #Business Process Governance #Change Management
Simon joined Carbogen Amcis in 2021 to accelerate the lab digitalization of the company and to leverage the business expert team to implement new corporate standards. With many years’ experience in operational and strategic leadership roles in the pharmaceutical industry, Simon provides hands-on knowledge on business process design & governance, ERP/LIMS & paperless lab initiatives as well as organizational change management and
business transformation.
Carbogen has been very ambitious in the domain of lab digitalization for many years. Their analytical labs in Switzerland are mostly paperless for more than 20 years now. Currently they are working on a global rollout of a new LIMS system across the different analytical labs in the sites.
In this presentation, we want to:
-
-
-
-
- Give an insight on the paperless lab achievements so far and give an outlook on the Carbogen Amcis LIMS roadmap ahead.
- Elaborate on ‘soft and hard’ key factors and learnings for project success
- Explore the benefits of agile business process design and discuss on the benefits of external expertise and benchmarks.
-
-
-
 Luis Dominguez, Director of Digital Transformation Group Operations at Lonza Visp
Luis Dominguez, Director of Digital Transformation Group Operations at Lonza Visp
#Artificial Intelligence #Workflow Automation
Dr. Domínguez is a Director of Digital Transformation Group Operations at Lonza. He is responsible for the strategy and execution of the digital transformation program in the areas of Technology Transfer and Testing. He leverages digital technologies such as Augmented Reality, Digital Twins, and Artificial Intelligence to increase operational efficiency, product quality, and safety and accelerate the time-to-market of pharmaceutical products. He is an expert in Industrial Automation and Control including Process Analytical Technology (PAT). Dr. Dominguez holds a Ph.D. degree from Imperial College London, the UK, and a MASc degree from the University of Ottawa, Canada, both in Chemical Engineering. He is currently completing an Executive MBA at IMD, Switzerland.
Dr. Gunnar Zoch, Innovation Manager at Lonza Visp
Mr Zoch focuses on digitalization projects in the QC labs with automation support, ranging from lab planning software implementations, programming apps for everyday use to worldwide prototype robotic projects.
This presentation will provide an overview of the innovative approach at Lonza Visp to automate the end-to-end workflow for water testing using the HoloLens and PyroTec® PRO Robot. 
-
-
-
-
- Implementing the HoloLens and AR technology for sample collection
- Automating the high throughput for Endotoxin texting using robotics
- Centralized data collection of all this information in a single system
-
-
-
 Mark Newton, Principal at Heartland QA ( Eli Lilly, retired)
#Data Integrity #Risk Management #Quality #People
Mark is an independent consultant who specialises in data integrity, laboratory informatics, computer systems validation, and Quality. He has 35 years of experience in the pharmaceutical industry in QC Labs, computer systems validation and lab informatics at Eli Lilly. Mark co-lead Eli Lilly’s data integrity remediation program for QC Labs worldwide in 2012, consulted and audited several Lilly sites preparing for data-integrity focused inspections.
Mark is a co-leader for the GAMP Data Integrity Special Interest Group and Chair of ISPE Global Documents Committee.
Co-author of "Harmonizing USP <1058> and GAMP for Analytical Instrument Qualification" Schuessler, Newton, Smith, Burgess, McDowall. Pharm. Engineering, Jan/Feb 2014. Co-editor of the GAMP Good Practice Guide "A Risk-Based Approach to Compliant Computerized Laboratory Systems" Nov. 2012.
Mark Newton, Principal at Heartland QA ( Eli Lilly, retired)
#Data Integrity #Risk Management #Quality #People
Mark is an independent consultant who specialises in data integrity, laboratory informatics, computer systems validation, and Quality. He has 35 years of experience in the pharmaceutical industry in QC Labs, computer systems validation and lab informatics at Eli Lilly. Mark co-lead Eli Lilly’s data integrity remediation program for QC Labs worldwide in 2012, consulted and audited several Lilly sites preparing for data-integrity focused inspections.
Mark is a co-leader for the GAMP Data Integrity Special Interest Group and Chair of ISPE Global Documents Committee.
Co-author of "Harmonizing USP <1058> and GAMP for Analytical Instrument Qualification" Schuessler, Newton, Smith, Burgess, McDowall. Pharm. Engineering, Jan/Feb 2014. Co-editor of the GAMP Good Practice Guide "A Risk-Based Approach to Compliant Computerized Laboratory Systems" Nov. 2012.
As an industry, we have taken the view that data integrity is a problem to be fixed. We look for activities and culture that contribute to data integrity and create countermeasures to reduce those risks to acceptable levels. But what if we have evidence that an organization has data integrity issues  because other parts of the organization have failed? What if data integrity is a pointer to a deeper root cause earlier in the process?
because other parts of the organization have failed? What if data integrity is a pointer to a deeper root cause earlier in the process?
This presentation will discuss a scenario where data integrity was a warning to look deeper into the control system. Along the path toward the root cause, we will examine how data integrity failures can point to places that may seem far removed from the source of the data integrity lapse. We will also discuss why these situations can easily destroy a culture where people are encouraged to “stand up and speak up” about quality issues.
 Presenter: Henry Farmery Associate Director of Informatics at Alchemab Therapeutics Ltd
Presenter: Henry Farmery Associate Director of Informatics at Alchemab Therapeutics Ltd

Henry Farmery is Associate Director of Informatics at Alchemab with responsibility for IT and software development at the company. Henry completed a PhD in Computational Biology at Cambridge University in 2017 and has subsequently worked in biotech startups with a focus on sequencing and bioinformatics. At Alchemab he led the implementation of the Benchling LIMS and continues to oversee its development as Alchemab build towards a paperless lab.
Early stage biotech companies are changeable entities. How do we implement a precise LIMS whilst developing the underpinnings of the platform itself? How do you balance the need for flexibility whilst working to capture mission critical data? This talk answers these questions in detailing the experiences of successfully implementing a cutting edge Benchling LIMS. We will examine the execution of these efforts and reflect on learnings a year on from the initial deployment.
 Igor Menghini, Head Software Product Management at Roche Diagnostics
Igor Menghini, Head Software Product Management at Roche Diagnostics
#Innovative Technologies #Digital Acceleration
Igor Menghini is a scientist who turned his attention to the world of data and software in the biomedical space; In his role at Roche he leads a groups of product managers and UX designers developing software for diagnostics applications. His love for science, technology, data, and the interplay of these 3  elements led him to look at the digital space under many different lenses.
elements led him to look at the digital space under many different lenses.
The word around us is evolving at incredible speed and companies need to embrace this evolution. In this talk Igor will share his experience in bringing different functions, process, and products into the digital world.
 Dr. Lloyd F. Colegrove, Consultant ( Retired Director of Data Services at Dow Chemical)
Dr. Lloyd F. Colegrove, Consultant ( Retired Director of Data Services at Dow Chemical)
#Real-time Analytics #Artificial Intelligence
Lloyd F. Colegrove recently retired as the Director of Data Services and the Director of Fundamental Problem Solving within Manufacturing and Engineering. He was also the Analytics Platform Director for Dow’s Manufacturing and Engineering’s Industry 4.0 program. Lloyd’s background is in Chemical Physics where he obtained a B.S. and Ph.D. from Texas A&M University. He spent 7 years in Polymer Research in Dow (R&D and TS&D) before moving into Manufacturing in an Analytical Improvement role and then a Quality Leader for five Dow businesses before moving to help establish a new capability called Fundamental Problem Solving, where top chemists and engineers work on complex, often multi-effect, plant problems. With this established, he refocused his efforts working to establish the data initiatives that support this work. Lloyd is married with two daughters and is an avid cyclist, hiker and landscaper.
The journey towards a major chemical company’s vision for the artful use of large structured and unstructured data sets in real-time plant operation and problem solving is a story of technology, salesmanship, and the hard work of convincing a company in love with Excel spreadsheets, and problem solving after-the-fact, that significant value lies in already collected and often under-utilized plant data sets. Part of the story includes the application  of artificial intelligence, but AI is not the solution, it is a solution. Through the use of volunteer audience participation and a few artful stories, this talk will discuss real-time analytics success stories that are actually simpler than “AI” infused solutions, discuss where AI fits in the chemical industry, and highlight some experiences from 30 years of engagement in chemical manufacturing that can bring tremendous bottom-line value to your chemical operations.
of artificial intelligence, but AI is not the solution, it is a solution. Through the use of volunteer audience participation and a few artful stories, this talk will discuss real-time analytics success stories that are actually simpler than “AI” infused solutions, discuss where AI fits in the chemical industry, and highlight some experiences from 30 years of engagement in chemical manufacturing that can bring tremendous bottom-line value to your chemical operations.
 Ulrich Markens, Global Head Quality, Business IT & Business Excellence at SGS
Ulrich Markens, Global Head Quality, Business IT & Business Excellence at SGS
#Global Implementation #QMS #Harmonisation Strategy
Ulrich Markens actually holds the position of Global Head Quality, Business IT and Business Excellence at SGS Life Sciences. He has more than 30 years experience in the Life Science and Health Care Industry.
After finishing University in 1985 he started his professional career at the Berlin University of Applied Science supporting Research activities and teaching Students in Physical Chemistry.
In 1988 he joined at a local Start-up Company in Berlin to lead and develop the environmental testing division. Within the next years the company grew exponentially, while broadening and diversifying the service portfolio into Pharmaceutical testing and Biotech services. After successfully establishing a robust Environmental division, Ulrich transitioned into the role of the Technical Director for the Pharma Division.
In 1999 Ulrich joined a previous Board Member composing a Business Plan for a Management Buy-Out (MBO) to establish PharmalytiCon, a dedicated Pharma CRO, providing Pharmaceutical Testing and Consultancy Services. Pharmalyticon was later acquired by Institut Fresenius and became part of SGS Life Sciences in 2006, as Center of Excellence for Method Development / Validation and Stability Studies.
In 2007 Ulrich beame part the SGS Life Sciences Global Management Team as VP / Regional Business Manger Asia based in Singapore. His mission was to lead SGS’ Life Science Services laboratories across Southeast Asia Pacific (India, Thailand, Singapore, China, Taiwan and Hong Kong), drive top line growth and profitability, implement best practices, drive harmonization and ensure compliance with local and Internal quality standards. During that time Ulrich consolidated SGS’ portfolio in Asia, grew top line significantly and turned around the business.
In 2011 Ulrich returned to Europe and took over the Global responsibility for Quality and Compliance. He established a Quality Governance structure encompassing a Global Quality Team, Internal Audit program, Global Quality Manual, Global SOP, Policies and associated KPI. Over the course of the next years the team grew sustainably, while Business IT were integrated.
Most recent development is the establishment of a Business Excellence Centre in Ireland providing centralized Commercial, Project Management and Business Application support to the entire Life Sciences network.

The presentation will describe the journey from converting a paper based QMS into a fully harmonised global system, established in 10 countries with more than 2'000 user. It will cover the supplier selection process, the challenges with the harmonisation, the planning workshops, deployment strategy and future roadmap and plans.
 Bob McDowall, director at R D McDowall Limited
Bob McDowall, director at R D McDowall Limited
#GMP Compliance #Data Integrity #Automated Lab
An analytical chemist with 50 years of experience, including working in the pharmaceutical industry for 15 years and afterward working for the industry as a consultant for 29 years. Bob has been involved with the automation of laboratories since 1980 and has over 35 years experience of validation of computerized systems and is the author of a book on the validation of chromatography data systems. His latest book is Data Integrity and Data Governance: Practical Implementation for Regulated Laboratories published in 2019 by the Royal Society of Chemistry. He is a member of the GAMP Data Integrity Special Interest Group, contributing to the Records and Data Integrity Guide in 2017 and two Good Practice Guides. Bob is also the author of the “Questions of Quality” and “Focus on Quality” columns in LCGC Europe and Spectroscopy magazines, respectively.
Reading current journal, scientific magazines and listening to presentations about automating a laboratory, you could be forgiven the assumption that many organisations are at the cutting edge of technology. However, when you see many medium and small sized laboratories nothing can be further from the truth:
 • Paper defined as raw data
• Paper defined as raw dataPANEL DISCUSSION COORDINATED BY ACCENTURE
 Mark Fish, Managing Director in Accenture’s Scientific Informatics Services Business.
Mark Fish, Managing Director in Accenture’s Scientific Informatics Services Business.
Mark has over 20 years of experience in various leadership roles delivering innovative solutions to the pharmaceutical sector and is passionate about drug discovery and development, translation research and precision medicine, digital transformation, agile software development and analytical quality control automation.
Peter Brandstetter, Senior Manager for Technology Consulting in Accenture in Zurich, Switzerland.
A respected authority in IT-enabled transformation in life sciences, he has more than two decades’ experience of solving complex IT challenges for pharmaceutical companies globally -gained across senior life sciences consulting/IT roles including Life Science Central Region Lead at CSC, Senior Managing Consultant for GBS Life Sciences at IBM, and Senior Manager at PwC.
Peter specializes in quality management and quality assurance in manufacturing and R&D, enterprise content management, and R&D (clinical data management, pre-clinical, R&D Lab, R&D collaboration and project management), as well as computer validation.
Jeremy Ward, Principal Director in Accenture’s Scientific Informatics Services Business.
Leader in Accenture’s Scientific Informatics Services practice in Europe with over 16 years experience in Scientific Informatics based in Base, CH and leading global client engagement and transformation. Jeremy specializes in working with organizations to transform their R&D, QA & QC digital and data management practices via risk management, process optimization and the implementation of innovative technologies.
read more in reflections-on-the-future-of-digital-in-the-laboratory/
WORKSHOPS SESSIONS
 Discover How Your Teams Can Get Work Done Seamlessly with Benchling
Discover How Your Teams Can Get Work Done Seamlessly with Benchling
While today’s science may be complex, Benchling’s suite of applications makes it easy to get work done together in real time. With an intuitive interface, scientists can structure and streamline work across teams, samples, products, locations, and the entire R&D lifecycle. In this workshop, we’ll highlight how teams can work together in Benchling to:
- Log experiments and protocols, collate information, and record results in shared, cloud-based Notebook
- Connect, contextualize, manage, and track samples, reagent vials, wells, batches and more with link to relevant results with Registry and Inventory
- Model and analyze biomolecule sequences with Molecular Biology
- Design, test, and optimize R&D processes with orchestrated process management with Workflows
- Extract meaningful, shared intelligence on operational and scientific success to make smarter decisions collectively with Insights
 The Lab of the Future - Today
The Lab of the Future - Today
What is the lab of the future? The answer will differ from one organisation to the next and where they are in the journey to achieve that vision will also vary.
In this workshop we will discuss the various components that will make up the lab of the future. What are the different technologies that will facilitate achieving this and how and where to start. We will also discuss the key considerations when evaluating new technologies to ensure that they meet your future needs and how to build a business case to show the return on investment and communicate the impact to the business.
 User Centric Lab Digitalization - Bring Real Value to the Lab
User Centric Lab Digitalization - Bring Real Value to the Lab
Imagine that data "automagically" follows you while working in the lab, in a non-invasive way. Data is transferred between instruments, ELN or LIMS but this does not require a big IT project or infrastructure. Sounds too good to be true? Join our workshop to find out.
 Actionable Lab KPIs: some industry best practices
Actionable Lab KPIs: some industry best practices
Measuring lab service levels, lab throughput, asset utilization, first time right, … Sounds straightforward, but extracting reliable and actionable key lab performance indicators is more complex than it sounds. In this 45’ workshop, we’d like to involve the audience and discuss some of the most critical questions:
- Which corporate lab KPIs are really important?
- How do you arrive at a shared definition of these KPIs? And should you harmonize those definitions across your lab network?
- How can you deploy those KPIs across multiple labs?
- How to collect the relevant data reliably and communicate the KPIs efficiently?
Of course, we’ll share our vision on each of these questions, some benchmark references, and best practices.
 Digitalize Your Experiment Not Your Paper!
Digitalize Your Experiment Not Your Paper!
A practical approach to manage the reproducibility crisis by means of experimental data digitalization
Who in the lab is constantly challenged by time-consuming documentation of laboratory procedures? Handling handwritten protocols that are loosely stored in a laboratory notebook can often result in loss of reproducibility. Full traceability of executed protocols and a gapless documentation of laboratory workflows are crucial for an efficient and productive lab environment.
 Drug Product Life Cycle Management - From Concept to Realization Overview
Drug Product Life Cycle Management - From Concept to Realization Overview
Let’s face it, the lab workload is testing compounds and samples. Lots of it! Increasing levels of digitization generates a wall of data; material specifications and laboratory methods to be set-up and maintained in many different Lab Systems amongst others. Global Value Web (GVW) deals with this data every day and proactively works towards an increased efficiency per Laboratory Master Data-type, so clients execute tests faster, reliably, with compliance, and preserving or contributing to the data integrity of the drug products involved.
 Ioi: a further step. Digitising laboratory workflows integrating instruments
Ioi: a further step. Digitising laboratory workflows integrating instruments
Last year Inpharmatic introduced Ioi as a platform for the Integration of instruments. Ioi is capable of solving Data Integrity issues without substituting actual (simple or legacy) laboratory instruments. Our platform electronically captures GMP data from isolated instruments and records the measurements in a central repository, including calibrations and daily checks. It also supports the review and the approval of each measure, and all the related instrument information, such as maintenance interventions.
This year we want to push Ioi one step further: supporting the digitalization of Lab workflows with or without the Integration of instruments.
 Accelerate your digital transformation with the most modern LIMS available
Accelerate your digital transformation with the most modern LIMS available
For every one of your lab activities. Toggling between applications creates complexity for users, stalling the momentum of digital transformation. We solved this problem with a LIMS built for the full scope of lab activities. Within a single platform, users can exchange workflows via an embedded ELN/LES; collect, secure, and store files and data created by instruments and other sources directly into the LIMS with SDMS; and use Analytics to visualize and mine lab and enterprise data.
 How to normalize scientific data for further use
How to normalize scientific data for further use
Automatization in laboratories calls for normalization of data from different instruments as the very first step. Proprietary data formats lock data in vendor or instrument specific software and make further usage of the data challenging. Converting such data into an open XML format like AnIML, makes the data available for usage (e.g., AI, ML, visualization) and archiving. We showcase how chromatography data from the most widely used software packages in chromatography can be converted in a single file format and compared with each other as well as with data from other instruments and technologies.
We grab data independent from instrument type or vendor from every data source, enrich it with available metadata and hand it over to any data consumer like ELN/LIMS/CDS etc. This gives users the opportunity to store or archive data and facilitates cooperation with internal and external co-operators.
 Digitalization and the data life cycle begin at the lab bench instruments
Digitalization and the data life cycle begin at the lab bench instruments
Full digital integration of processes is crucial for a successful laboratory, as handwritten records are time consuming and prone to errors.
Laboratory managers and operators routinely face challenges to adhere to data integrity regulations, while aiming at increasing workflow efficiency. Adding the technical controls to instruments improves business processes at the same time it adds the necessary controls for data governance.
Regulators are increasingly requiring labs to get current with technology (the "c" in cGMP). Regulatory guidance from WHO even advocates process efficiency with technology. The benefits of managing data in a fully electronic process provides business benefits and regulatory compliance with data stored in one medium. This approach facilitates efficiency and effectiveness including ease of performing data analytics.
Don’t just digitalize, and don’t try to make a digital system work "the old paper way", make sure you get real business benefit from your digitalization efforts by really improving the workflow in the lab.
 Unlocking Lab Efficiency through Digital Transformation: Signals Research Ecosystem
Unlocking Lab Efficiency through Digital Transformation: Signals Research Ecosystem
Research Scientists daily face the challenge that the science they are doing is complex and often generates a high volume and variety of data. In addition, they are spending too much time preparing this data and learning the tools to explore it vs the time they can spend on ideation and innovation. In this workshop we will present the PerkinElmer Signals Research Platform which is designed from the ground up to consider these challenges.
Agenda topics will include how Signals Research Platform can be used to analyze instrument data coming from any R&D workflow whether it is derived from a plate reader or an analytical instrument. Furthermore, we will demonstrate how the experimental context can be captured and used for further downstream analysis including combining this with other inhouse or public data sources to facilitate better decision making. Join us in our workshop and see for yourself why the Signals Platform has been selected by some of the leading pharma and chemical companies including GSK, Bayer, Janssen.
 The Productivity Paradox of Quality 4.0
The Productivity Paradox of Quality 4.0
 Achieve complete laboratory orchestration through digital and technological innovation
Achieve complete laboratory orchestration through digital and technological innovation
Complete laboratory orchestration is critical in moving organizations forward on their digital transformation journey. Although the goal is the same, each organization has its own complex and tailored scientific ecosystem, as well as its own unique set of processes and challenges. Leveraging innovative digital tools, technology, and software solutions will create a seamlessly connected ecosystem that will ultimately eliminate those challenges, enhance operations and improve efficiency throughout. Full connectivity provides enormous benefits to a laboratory – allowing scientists to ultimately focus on the science and drive scientific discoveries.
>> see www.paperlesslabacademy.com/sponsors-thermofisherscientific
*agenda subject to changes


