The PLA2022 India is finally in person at Novotel Hyderabad Convention Centre
It is an opportunity for visitors and providers to re-connect with human interactions, after so many video-calls, webinars and virtual events.
 We are building a great program for great discussions. Scroll down and discover the Topics and the Speakers
We are building a great program for great discussions. Scroll down and discover the Topics and the Speakers
In association with EMINENCE BUSINESS MEDIA see press release announcement
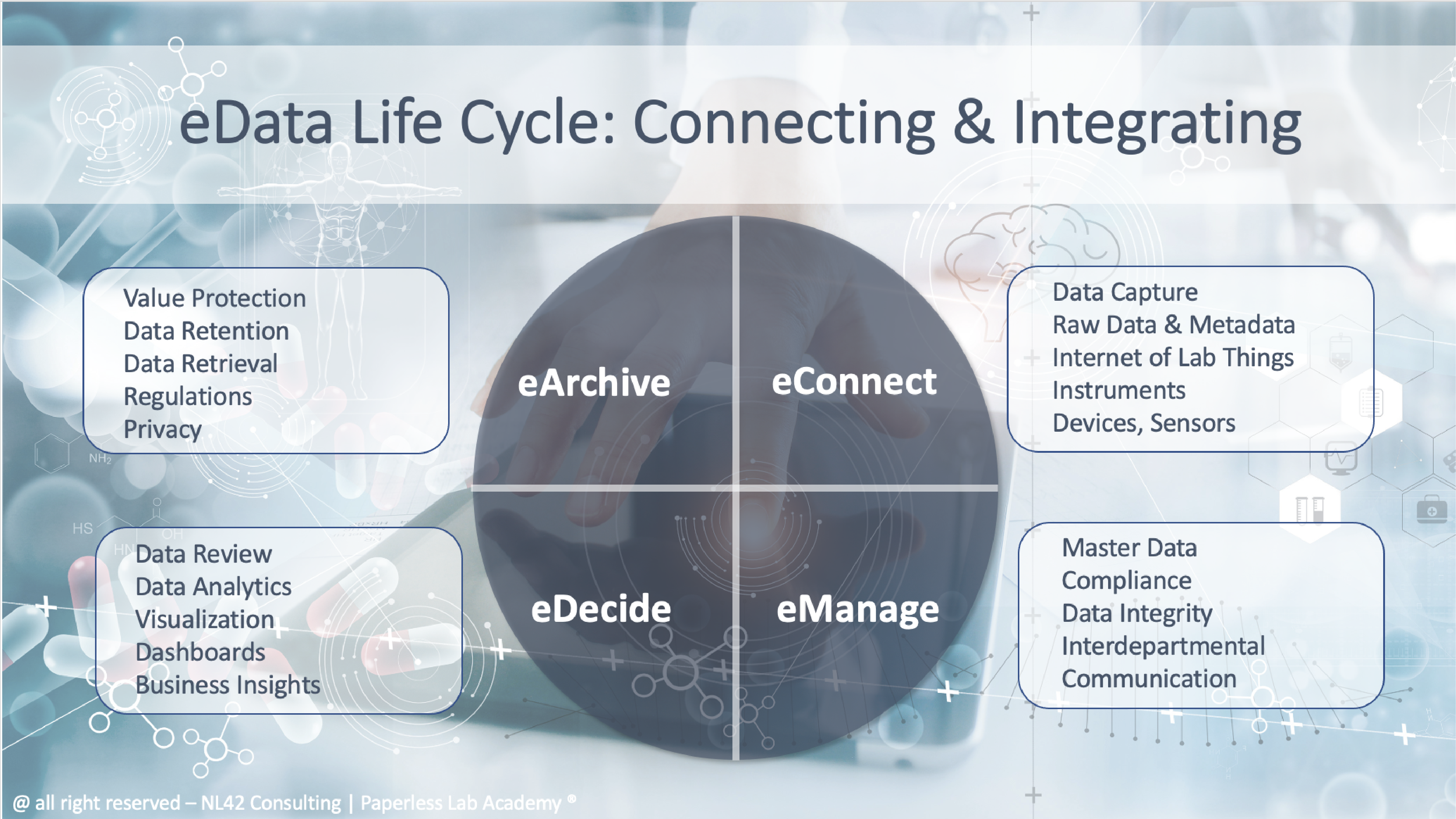
eData Lifecycle Discussions
Digital data have a specific lifecycle from their creation to final storing. At every step those data should be easily reachable and bring the necessary information to facilitate the decision making. Industry compliances also matter here and need to be covered. Yet above all the right management of those data is crucial for its company business.
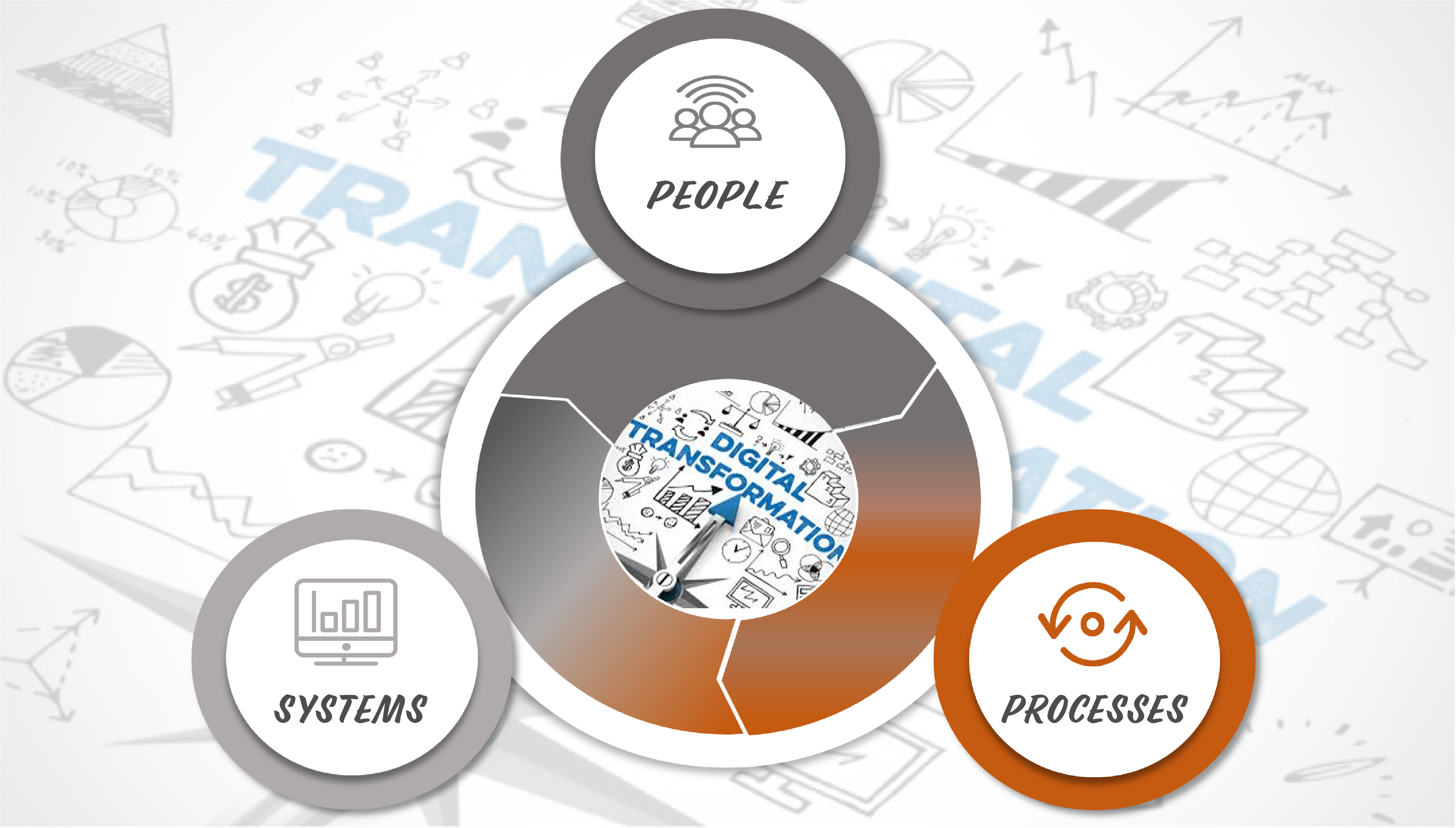
Driving the Change Management
Managing a Digital Transformation project in your company for your laboratory and quality processes is not a flawless project. Beside Your processes and the adequate systems to be selected and implemented, every one that have been through it will always reinforce the point that getting your team on board is crucial. The Human Factor is key for a successful digital transformation project.

Digital Data Management Topics
Topics covered are about Digital Data Management all along the eData lifecycle and all the related matters such as data integrity, data integration, data analytics, data standardisation, structured vs unstructured data, data search, machine learning, artificial intelligence, etc.
See below PLA2022 India Program content

 Isabel Muñoz-Willery Ph.D. & Roberto Castelnovo, owners & organizers at NL42 Consulting | Paperless Lab Academy®
Isabel Muñoz-Willery Ph.D. & Roberto Castelnovo, owners & organizers at NL42 Consulting | Paperless Lab Academy®
Isabel and Roberto are entrepreneurial persons involved in paperless laboratory processes projects. Coming from international managerial roles with strong domain knowledge in laboratory processes, laboratory automation, laboratory informatics, and project management, they launched their personal project, NL42 consulting, by 2012. Soon their own customers were asking for better visibility of the industry solutions and learn about the latest technological trends and best-recommended methodologies.
Along the time, the Paperless Lab Academy® has become the platform where to find the answers. In 2023, the PLA European congress will celebrates it 10th edition.
 The PLA is now expanding and sharing the experience in India and US.
The PLA is now expanding and sharing the experience in India and US.

Sachin Bhandari, Sr. General Manager, Global Head QA-IT & Quality Digitisation Projects at SunPharma
#ISPE India #GAMP #Quality #CSA
Sachin is a Seasoned professional with over 20 years of experience in Pharmaceutical and healthcare compliance domain with wide experience in GXP, 21 CFR part 11, Annex -11, GAMP 5 compliance (Validation and Quality). He has primarily worked in in Validation activities related to Quality systems, Lab Systems Validation, IT Infrastructure Validation, and Supply chain Validation, Quality systems and service desk compliance. He currently leads a Pharma Generics Major where is driving paperless initiatives, implementation of technological innovations to improve the quality and compliance.
Sachin is Chairman of ISPE GAMP steering committee India Chapter and is prominent speaker at various forums and seminars.
 Sanjay Nandavadekar, Director IT at Cipla
Sanjay Nandavadekar, Director IT at Cipla
#Paperless Initiatives #Business Process Design #Business Process Governance #Change Management
Sanjay is a passionate IT executive with 20+ years of experience in the Manufacturing -Pharmaceutical industry. His strategic leadership and certified skills have helped organizations to achieve greater automation, efficiency improvements, and technology leadership using technology as a catalyst. Sanjay has a proven track of leadership expertise in crafting the digital strategy for a large, global enterprises. Sanjay is leading a portfolio of cutting-edge technologies like Industry 4.0 (AI, ML, IoT, RPA, Chatbot, NLP, BI, and Cloud) for enterprise applications. He has architectured mega technology projects from strategy to implementation and, let them for greater adoptions. He has demonstrated strategic and financial skills by owning high cost budgets. His business relations with industry leaders, vendor ecosystem, and peer CIO network are helping him to stay abreast of technology trends. Sanjay is a well-recognized speaker in the industry and has received many recognitions, awards on various platforms.
![]() Sanjay will cover the paperless lab journey from concept to adoption. Typically my focus would be bringing a high level of process for implementing lab solutions right from RFP to change management and various aspects of challenges and opportunities in this area.
Sanjay will cover the paperless lab journey from concept to adoption. Typically my focus would be bringing a high level of process for implementing lab solutions right from RFP to change management and various aspects of challenges and opportunities in this area.
 Manish Kumar, Head of Enterprise Architecture, Global Drug Development at Novartis
Manish Kumar, Head of Enterprise Architecture, Global Drug Development at Novartis
#Enterprise Architecture #IT Strategies #Customer first
Mannish is a Life Science and Healthcare Technology leader with wide experience in transforming the IT and aligning the technology elements to the business roadmap. worked with fortune 100 tech companies, and start-up business.
Manish has experiences in handling complex technology transformational programs with effective strategies in the area of Enterprise Architecture, Product Engineering, Technology modernisation, and Enterprise data strategy. Has differentiated himself by building Product. Platform and Solutions strategies around customer-first, feature-driven rollouts, and data agnostic tech stack.
![]()
 Mrs Neeru Bakshi, Founder at Tech Qualitas
Mrs Neeru Bakshi, Founder at Tech Qualitas
#CLOUD #Validation #Regulations
Regulatory and technical software is nothing without a team of data and science experts at its core. Neeru is one of those ultra-valuable veteran data experts that digs into the nitty gritty of regulations, guidelines, and systems and makes sure the organization is up to date and on track with any new technical developments in QA, validation or any other industry standards.
Neeru Bakshi has more than with 20 years’ experience in the pharmaceutical and Life Sciences domain. She worked as Validation/QA Lead, Project Manager/Lead, and as Business Analyst for various cloud solutions and systems such as Oracle Life Sciences Applications for Clinical Trails, SAP, GLP systems, Electronic Submissions, Pharmacovigilance Systems.
Neeru is well versed in regulatory standards and guidelines; 21 CFR Part 11, Computer System Used in Clinical Trials, GAMP5 guidelines, EudraLex Volume 4 Annex11 along with knowledge of European data protection laws and practices and understanding of the GDPR. She has experience and interest executing harmonization, development and implementation of Global Quality and IT/CSV Policies/ SOP’s/ Guidelines across the organization level. She also has experience in the pharmaceutical industry audits of validated computer systems, and supporting clients in such pharmaceutical industry audits, whether they be internal audits, sponsor-driven audits, or regulatory agency audits.
 Cloud solutions have come of age and have tremendous potential as it provides resilience, security, and scalability it confers—and all with speed and at low cost for implementation and maintenance. Leading pharmaceutical and life-sciences companies are discovering the potency of cloud in enabling analytics, shrinking innovation cycles, and standardizing processes across global operations, among other benefits. During COVID pandemic, pharmaceutical companies could deliver COVID-19 vaccine in shorter time using cloud technology since it does not require reinvention and allows to fly limitless.
Cloud solutions have come of age and have tremendous potential as it provides resilience, security, and scalability it confers—and all with speed and at low cost for implementation and maintenance. Leading pharmaceutical and life-sciences companies are discovering the potency of cloud in enabling analytics, shrinking innovation cycles, and standardizing processes across global operations, among other benefits. During COVID pandemic, pharmaceutical companies could deliver COVID-19 vaccine in shorter time using cloud technology since it does not require reinvention and allows to fly limitless.
Although belief in the value of cloud is widespread, a clear understanding of maintaining validated and controlled state of cloud solutions by solution provider and users (pharma and life-sciences companies) state of precisely how much required from provider and users—and how to capture it—is often lacking, leading to misguided strategies and faulty implementation or too much validation and documentation; sometimes repeating everything which cloud solution provider has already executed.
This session will cover topics such as but not limited to:
- Cloud Solutions Overview
- Myths about Cloud Solutions
- Benefits of using Cloud Solutions
- Maintaining Validated State – Provider and Users
 Mr. Raj Nandhan, Director, Quality & Compliance at Medocity Inc.
Mr. Raj Nandhan, Director, Quality & Compliance at Medocity Inc.
#DataQuality #GXP #KPIs
![]()
 Mr Rajesh Thempadiyil, Head- Quality Digital Transformation at Dr. Reddy´s Laboratories
Mr Rajesh Thempadiyil, Head- Quality Digital Transformation at Dr. Reddy´s Laboratories
#LIMS Project #Digital Transformation
Rajesh T has over 20 years experience working in pharmaceutical industry. He has handled roles in his career namely, Quality Management Systems, Regulatory Compliance, Validation & Qualifications and Computerized System Validation with different organizations. In the current role he is responsible for Digital Transformation in Manufacturing and Quality Operations at Dr. Reddy’s and he is part of Digital & Process Excellence team. He is a core team member in the ISPE GAMP Good Practice Guide on “Data Integrity – Manufacturing Records” guide. He has authored chapters in the subjects of aseptic processing and Manufacturing Execution Systems. He is part of ISPE and acted as Co-Chair for GAMP India CoP. In ISPE he is working with SIG on Data Integrity, SIG on Pharma 4.0, SIG on Manufacturing Execution System and GAMP Awareness.

 Sreedhara Rao Abburu, Senior Deputy General Manager – QC at Divi’s Laboratories Limited
Sreedhara Rao Abburu, Senior Deputy General Manager – QC at Divi’s Laboratories Limited
#LabInformatics #Paperless Processes #Systems Interface
Abburi Sreedhar Rao is a prolific quality control enthusiast with over 25 years of experience in quality control operations. He holds a Master of Science degree in Applied Chemistry and achieved a Ph.D. in Analytical Chemistry. With substantial knowledge of the regulatory guidelines and industry exposure, Sreedhar has successfully handled many regulatory inspections from different authorities around the globe. He plays a vital role in enhancing staff performance, developing/implementing new processes, and leading high-performing teams.
Sreedhar has vast experience on instrument calibration, software troubleshooting, LIMS implementation, and application software implementation. He is a versatile contributor to all quality control activities and release of API products. Having sound experience in gap analysis of computerized systems, he has also worked on CSV with consultants from global organizations.
Sreedhar worked for 25 years with Divi’s Laboratories Limited and had a key role in Analytical Method Development Support and Quality Control Operations. Now, he is all set to lead the Quality Control operations in Divi’s.

The panel discussion is designed to give participants the opportunity to interact proactively with the keynote speakers from the industry.
Mr Bhandari from Sun Pharma, Mr Nandavadekar from Cipla and Mr Kumar from Novartis will openly share their experiences and personal do&do not list for those thinking about consolidating, integrating and simplifying scientific data management systems with the audience.
#Project Implementation #Change Management #Technologies #KPIs
The panel discussion is designed to give participants the opportunity to interact proactively with the speakers from the industry.
Raj Nandhan from Medocity Inc, Mrs Neeru Bakshi from Tech Qualitas and Roberto Castelnovo from NL42 will discuss about the challenge of being compliant today while considering newer technologies for tomorrow
#CSV #DataQuality #Cloud
The panel discussion is designed to give participants the opportunity to interact proactively with the solutions and services providers from the industry.
Sekhar Surabhi from Caliber Technologies, Mark Gonzalez from LabWare and Pradeep Nagisetty from Veeva Systems will share their vision about the new technologies to be seen in a near future and how those will apply to the market needs.
#CLOUD #AI #ML #AUTOMATION #PREVENTIVEACTION
WORKSHOPS SESSIONS
 How to Achieve High Productivity and Compliance with a Robust LIMS
How to Achieve High Productivity and Compliance with a Robust LIMS
When labs undertake digital transformation, they look for ROIs like compliance, efficiency, profitability, etc. A robust LIMS system can ensure that these ROIs are met effectively.
Having led the implementation of India’s First 100% Paperless Lab, Rajasekhar talks about committing to the Paperless Lab vision and achieving high productivity and compliance with a robust LIMS.
In this workshop, we discuss how a LIMS helps in productivity and compliance, how to ensure that the lab is chasing the right ROIs, and how to make the best use of a LIMS.
>>> see more at paperlesslabacademy.com/sponsors_caliber
 Pharma R&D to Manufacturing – Importance of Digital
Pharma R&D to Manufacturing – Importance of Digital
Continuity
DASSAULT SYSTEMES helps Research Labs/Process Development Labs/CROs/CMOs/CDMOs achieve Collaborative Innovation & Operational Excellence through Digital Continuity. Going Paperless with BIOVIA solutions is the key that helps organizations attain Digital Continuity. Discover its latest offering, presented in an interactive workshop.
>>> see more at paperlesslabacademy.com/sponsors_DSBiovia
 A collaborative data management tool for chemical and biological therapeutics
A collaborative data management tool for chemical and biological therapeutics
A standardized tool is very much in demand to manage the big dataset and share it with collaborators. In addition, there is a growing need to securely integrate public data with private data. Hence, here we present the cloud-based data management tool Collaborative Drug Discovery (CDD) Vault that provides a more collaborative hosted informatics technology for many commercial and drug discovery applications. The major components of effective scientific-community based research include:
- unifying goal or focus on common therapeutic areas/diseases
- multiple research areas/expertise
- uniform database platform for effective data accumulation and management
- easy access and sharing of information
- potential for unlimited growth
Essentially, new approaches that would allow scientists to do efficient research in order to handle registration of complex chemicals (stereochemistry, mixtures, synthetic conjugates) and biologicals (nucleotides, amino acids and complex drug-like biological entities i.e., Antibody drug conjugates, PROTAC, molecule Glue) as well as assay data around these entities, all can be covered using CDD Vault.
>>> see more at paperlesslabacademy.com/sponsors_CDD
 Life Sciences Value Chain Data Backbone of the Future: From Concept to Realization Overview
Life Sciences Value Chain Data Backbone of the Future: From Concept to Realization Overview
A complex life sciences environment demands a resilient value chain and transformed business models that break down data silos, reduce turnaround times, and place patients at the center of your operations. Traditional value chains generate decentralized footprints, leading to a clustered enterprise that lacks transparency between customers and stakeholders.
With an industry-wide connected value chain, data is readily available and accessible, which enables informed decision-making and quick reactions to disruptions in the supply chain. This also contributes to data integrity and on-time delivery of quality products.
>> see more paperlesslabacademy.com/sponsors-globalvalueweb/
 Digitalization, The Lab of the Future and Lab 4.0
Digitalization, The Lab of the Future and Lab 4.0
The Lab of the Future (Lab 4.0) is the collective name for the technologies and processes that will enable the next generation of scientists and researchers.
In this session, you'll learn about the trend of the future labs, how Technology will transform Laboratory and the challenges and strategies to consider moving forward.
>> see more paperlesslabacademy.com/sponsors-labware/
 Practical applications of Data Integrity and Audit Trail Review
Practical applications of Data Integrity and Audit Trail Review
The why, what, when, where, who and how of audit trail review. In this session you'll learn about regulatory requirements around audit trail review, examples of audit trail review strategies and roles for audit trail review.
>> see more paperlesslabacademy.com/sponsors-lonza/
 The Productivity Paradox of Quality 4.0
The Productivity Paradox of Quality 4.0
*agenda subject to changes