#PLA2020India Program: Foster Digital Revolution in your Lab
The Paperless Lab Academy® is the ideal learning platform for all companies that own a laboratory.
Everything you always wanted to know about consolidating, integrating or simplifying laboratory data management processes.
Once again, we´re setting the stage for discussions on strategies and implementation of 21st-century technologies in your laboratory.
We offer an agile combination of future horizon views - already running in some industries - and tangible real cases on how to solve today’s concerns.
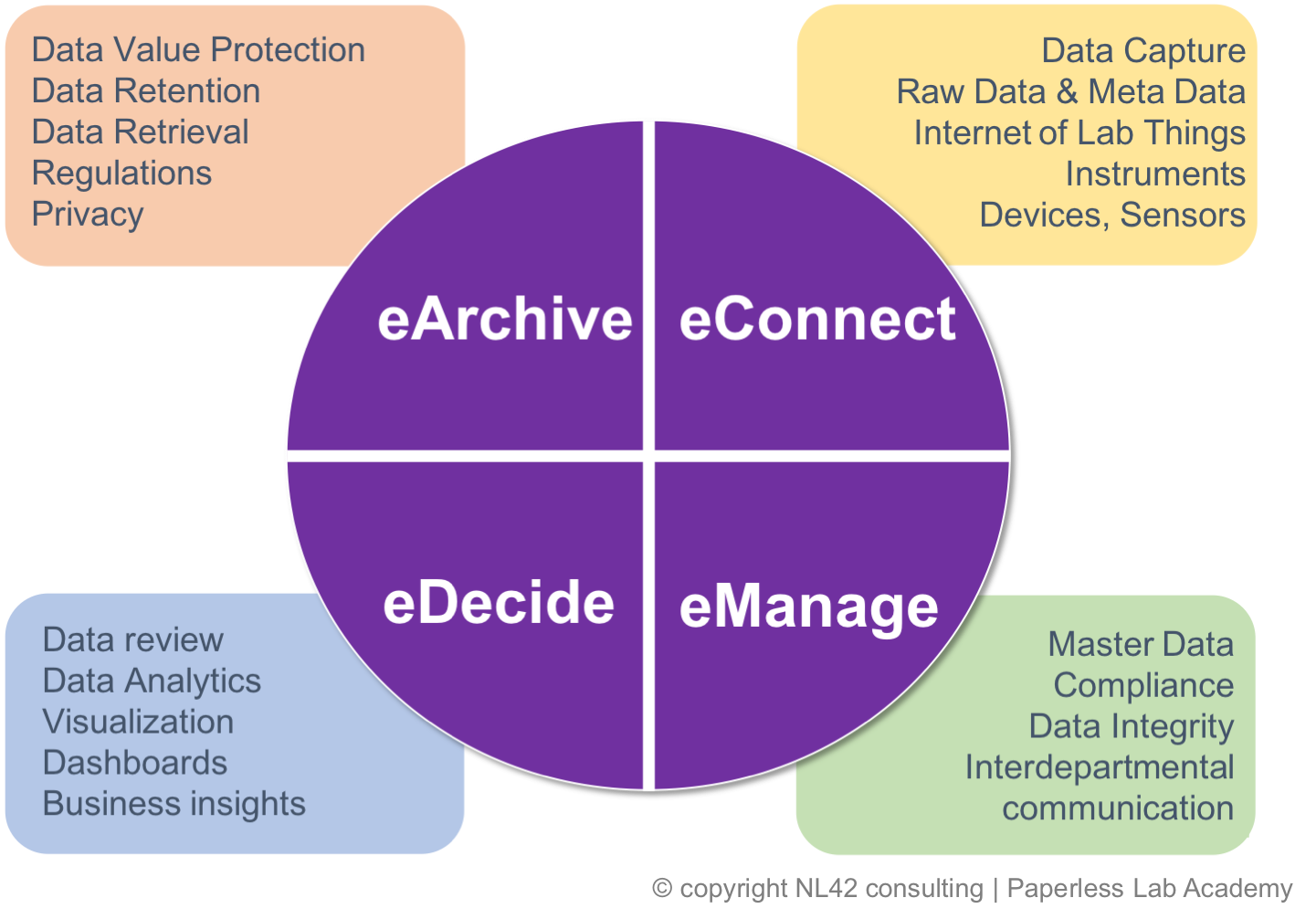
WHAT WILL YOU LEARN
- About the future of the laboratory within the industry 4.0: Artificial Intelligence
- Experiences during COVID situation: IT Strategies
- The relevance of mastering your data for efficient scientific data management
- The reality of the #IoLT, Internet of Lab Things and #Ioi, Integration of instruments
- FAIR principles, Data analytics
- Why archiving is the beginning and not the end of a solid #eDataLifeCycle
- Practical sessions on Audit trails, Data Integrity, and new Compliance guides
- Demystification of the Cloud-based solutions
- How to successfully implement laboratory system solutions
WHO SHOULD ATTEND
Industries that significantly benefit from the Paperless Lab Academy® congress include: Pharmaceutical, Lab Services, Biotech, Food & Beverage, Consumer Goods, Cosmetic, Chemical, Oil & Gas
People visiting us every year range from Laboratory Directors and Managers, Operational Excellence, IT Business Partners, QC, and QA Management, Research departments, and Manufacturing
PLA2020 INDIA PROGRAM
Dec 1-3, 2020

 Presenting: Isabel Muñoz-Willery Ph.D. & Roberto Castelnovo, owners & organizers at NL42 Consulting | Paperless Lab Academy®
Presenting: Isabel Muñoz-Willery Ph.D. & Roberto Castelnovo, owners & organizers at NL42 Consulting | Paperless Lab Academy®
#Scientific Data Management #eData Life Cycle #Data Master Plan
Isabel and Roberto are entrepreneurial persons involved in paperless laboratory and quality processes projects. Coming from international managerial roles with strong domain knowledge in laboratory processes, laboratory automation, laboratory informatics, and project management, they launched their personal project, NL42 consulting, by 2012. Soon their own customers were asking for better visibility of the industry solutions and learn about the latest technological trends and best-recommended methodologies.
The Paperless Lab Academy® has become the platform where to find the answers, about consolidating, integrating or simplifying laboratory data management processes.
 Presenter: Sergio Nasi, Head of IT Operations Laboratory Execution at Boehringer-Ingelheim
Presenter: Sergio Nasi, Head of IT Operations Laboratory Execution at Boehringer-Ingelheim
#LabInformatics #Paperless Processes #Systems Interface #Pandemic Situation

 Presenter: Govi Sridharan Associate Director, Global Head of Pharma Technical Development Business Operations Informatics at Genentech
Presenter: Govi Sridharan Associate Director, Global Head of Pharma Technical Development Business Operations Informatics at Genentech
#Innovative Technology #Holistic approach to Data #BigData
Govi has move than 20 years experience in digital transformation and automation projects.
He's actually the Digital Transformation Enablement lead for global delivery of solutions using Google Cloud Platform for PTD organization.
>> see the abstract about conceptual architecture for digitalised laboratories

 Presenter: Vipul Doshi, President - Group Quality Assurance and Regulatory at Zydus Cadila
Presenter: Vipul Doshi, President - Group Quality Assurance and Regulatory at Zydus Cadila
#Digital Journey #Data Insights #Business Intelligence #Scalable Productivity
Mr. Vipul Doshi is the President - Group Quality Assurance and Regulatory at Zydus Cadila. He is an accomplished pharmaceutical professional serving the Pharmaceutical Industry from more than three decades. He has led the Quality and Regulatory affairs function in various Pharma Companies of high credentials. Throughout his career he has provided passionate global leadership and pioneered in guiding the company to meet the Global Regulatory, Quality Standards and Compliance requirements.
He is a leading advocate for Global Competitiveness and instrumental in due diligence for various acquisitions and mergers. He possesses great expertise in Projects and Engineering for developing Infrastructures for APIs and Drug Products (Sterile and Non-Sterile). He is actively involved with various professional associations like ISPE/PDA/AAM.
>> see more about building a robust paperless laboratory with integrated quality approach

 Andreas Steinle, Manager Digital Solution at Roche Diagnostics GmbH
Andreas Steinle, Manager Digital Solution at Roche Diagnostics GmbH
#Case Study #Data Transfer #FAIR principles #SCRUM #Cloud #GXP
Andreas Steinle is accountable for digital transformation in the analytic department of Roche Pharmaceuticals Technical Development Europa since early 2018.
Between 2005 to 2017, Andreas was head of the group “Analytical Systems” with the responsibility for support of validation of analytical systems and laboratory equipment (pH Meter, HPLC, LIMS, Archiving, etc.) in Roche Pharma Biotech Production in Penzberg.
In addition in this role he he was also responsible for the integration of laboratory equipments into enterprise systems and the representation to health authorities.
>> see the abstract about Unlock your analytical instrument data for data science


Presenter: Ms. Preeti Gaonkar, SEAP Quality Excellence Manager at SGS India Private Limited
#User Case #Quality Management System #Cloud #Electronic Workflows

 Presenter: Dr. Lloyd F. Colegrove, Director of Data Services at Dow Chemical
Presenter: Dr. Lloyd F. Colegrove, Director of Data Services at Dow Chemical
#Industry4.0 #Artificial Intelligence #Digital Transformation Strategy #Change Management
Lloyd F. Colegrove recently retired as the Director of Data Services and the Director of Fundamental Problem Solving within Manufacturing and Engineering. He was also the Analytics Platform Director for Dow’s Manufacturing and Engineering’s Industry 4.0 program. Lloyd’s background is in Chemical Physics where he obtained a B.S. and Ph.D. from Texas A&M University. He spent 7 years in Polymer Research in Dow (R&D and TS&D) before moving into Manufacturing in an Analytical Improvement role and then a Quality Leader for five Dow businesses before moving to help establish a new capability called Fundamental Problem Solving, where top chemists and engineers work on complex, often multi-effect, plant problems. With this established, he refocused his efforts working to establish the data initiatives that support this work. Lloyd is married with two daughters and is an avid cyclist, hiker and landscaper.
>> see more about Digital Transformation in the Chemical Industry. The Devil's in the Details.

 Presenter: Mr. Dinu Abraham, Senior Manager – Information Technology at Lupin Limited
Presenter: Mr. Dinu Abraham, Senior Manager – Information Technology at Lupin Limited
#R&D #Driving Innovation
Over 15 years of thought leadership expertise in effectively partnering with the business, stakeholders and product development team to manage large scale Global IT Transformation / Digitization Programs in Highly Regulated Environment– Implementation of GxP Applications, IT Business Applications (cGMP / GxP Software, SOX & other commercials applications) Portfolio Management, CSV Lifecycle / Program Quality Management, Business Process Re-engineering, QC Lab & Mfg. Shop floor Automation, Setting up of CoE, Product Development/Enhancement.
>> see the abstract: Digital Platform for R&D

 Presenter: Toni Manzano, Chief Science Officer, R&D Director and Co-founder at Bigfinite
Presenter: Toni Manzano, Chief Science Officer, R&D Director and Co-founder at Bigfinite
#Artificial Intelligence #Big Data #DataAnalytics #Statistics #NewRegulations
Toni is the co-founder and CSO of Bigfinite, a cloud company that provides big data and AI SaaS platform for the Biotech and Pharma industry. He is also member of the scientific committee of the PDA Europe, Lead of the AI in Operations team for the AI Xavier University and teaches AI subjects at the University (UAB), member of the Science Experts in the Spanish Parliament on big data and artificial intelligence topic. He has written numerous articles in the Pharma field and holds a dozen international patents related to the encryption, transmission, storage and processing of large volumes of data for regulated environments in the cloud.
Toni is Physicist, Master in Information and Knowledge Society and post graduated in quality systems for manufacturing and research pharmaceutical processes.
>> see the abstract: Data Centricity - What is behind the AI implementation in Pharma


Planning the Next Move – How to Become Future Ready with Integrated Quality Management
#Quality Management #Roadmap Digital Transformation
Presenting: Sumanth Chinta, LIMS Subject Matter Expert, Head of Presales at Caliber Technologies
Sumanth Chinta has been working in Caliber for more than 13 years, having the opportunity to work with major industry leaders in the Pharmaceutical, Petrochemical and Life Science industry in the USA, UK, Singapore, and India. There he has taken up multiple roles that have given him the opportunity to implement and perform technical pre-sales and sales of ‘Laboratory Informatic Systems’ across the globe.
 Digital Continuity and Documented Compliance with BIOVIA ONE Lab
Digital Continuity and Documented Compliance with BIOVIA ONE Lab
#Innovation #Harmonisation #Standardisation
Presenting: Dennis Curran, Director – Product Management, Unified Laboratory Management at Biovia
Dennis Curran is the Portfolio Lead of the Experimental Decisions Group for Dassault Systemes BIOVIA and is focused on the integration of BIOVIA products into customer solutions for scientific research and development. The areas of product responsibilities include BIOVIA Laboratory Solutions and mapping the product transition to 3DEXPERIENCE. Mr. Curran has direct pharmaceutical experience while running the robotics laboratories for Parke-Davis’s Analytical Development department in New Jersey.
 Durable Data – Securing Your Instrument Data
Durable Data – Securing Your Instrument Data
#Data Capture #Data Protection #Scientific Data Management system
Presenting: Charlie Wakeham, APAC GxP Compliance Manager at Waters Corporation
Charlie Wakeham has more than 20 years of industry experience developing and validating computerised systems for regulated production and laboratory environments. As Waters Corporation’s GxP Compliance Manager, she provides practical and pragmatic computerised systems validation assistance and data integrity consultancy to regulated companies implementing Laboratory Informatics in Asia Pacific (APAC) countries.
>> see the abstract of Waters Workshop: durable data - securing your instrument data
 The Connected lab – How digital transformation is changing the way we do science
The Connected lab – How digital transformation is changing the way we do science
#Integrated Ecosystem #Productivity #Efficiency #Connectivity
Presenting: Sudhakar Kasturi, Senior Product Specialist and Digital Science at Thermo Fisher
Sudhakar Kasturi is a business leader with 25 years of broad based experience in Life sciences, Petroleum, Food Safety, Chemical, environmental , basic research, analytical and laboratory industries with a strong track record of success in consistently delivering robust sustainable profitable sales growth.
>> see the abstract of Thermo FisherWorkshop: The Connected lab
 Digital Solutions to meet Data Integrity & Compliance
Digital Solutions to meet Data Integrity & Compliance
#Data Integrity
Presenting: Mukunth Venkatesan, CEO at Agaram Technologies
Mukunth Venkatesan started his career as a bio-medical instrumentation engineer.
He joined Agaram group 26 years back to lead the analytical instrumentation and R&D division for manufacturing HPLCs in India.
Mukunth moved on to establish a software development team for instrumentation. Once he cut his teeth in instrument software development, the next logical step was to start developing “Laboratory Informatics” software.
>> see the abstract of Agaram Workshop: Digital Solution to meet Data Integrity and Compliance
 MODATM is a comprehensive platform for environmental, utility and product monitoring, combining automated scheduling, workflows, mobile data acquisition, device integration, and visual analytics.
MODATM is a comprehensive platform for environmental, utility and product monitoring, combining automated scheduling, workflows, mobile data acquisition, device integration, and visual analytics.
It eliminates paper-based monitoring and testing that can be expensive, error-prone, time and labor- intensive, therefore enhancing Data Integrity, reducing timelines and potentially saving clients QC costs.
 Presenter: Bob McDowall, director at R D McDowall Limited
Presenter: Bob McDowall, director at R D McDowall Limited
#Chromatography #CDS #LIMS #ELN
An analytical chemist with over 45 years’ experience including 15 years working in the pharmaceutical industry and over 25 years working for the industry as a consultant. Bob has been involved with automating analytical laboratories since 1980 and was an early implementer of a Laboratory Information Management System (LIMS).
Bob is an auditor and he has been involved with the validation of computerized systems for over 30 years and is the author of books on the Validation of Chromatography Data Systems (two editions 2005 and 2017) and in 2019 published his book on Data Integrity and Data Governance - Practical Implementation in Regulated Laboratories.
>> see abstract about if spreadsheets are a fast track to regulatory non compliance
 Presenter: Mark Newton, Principal at Heartland QA
Presenter: Mark Newton, Principal at Heartland QA
#Data Integrity #Metadata #Chromatography
Mark is an independent consultant who specialises in data integrity, laboratory informatics, computer systems validation, and Quality. He has 35 years of experience in the pharmaceutical industry in QC Labs, computer systems validation and lab informatics at Eli Lilly. Mark co-lead Eli Lilly’s data integrity remediation program for QC Labs worldwide in 2012, consulted and audited several Lilly sites preparing for data-integrity focused inspections.
Mark is a co-leader for the GAMP Data Integrity Special Interest Group and Chair of ISPE Global Documents Committee.
Co-author of "Harmonizing USP <1058> and GAMP for Analytical Instrument Qualification" Schuessler, Newton, Smith, Burgess, McDowall. Pharm. Engineering, Jan/Feb 2014
Co-editor of the GAMP Good Practice Guide "A Risk-Based Approach to Compliant Computerized Laboratory Systems" Nov. 2012.
 Presenter: Vipul Krishan Yamdagni, Independent GMP consultant and advisor
Presenter: Vipul Krishan Yamdagni, Independent GMP consultant and advisor
#Systems Integration #Digitalised Processes #Scientific Data management
Vipul Krishan Yamdagni is a seasoned Quality Assurance professional with a strong analytical frame of mind and a result-oriented approach.
He has spent over 34 years in the industry with major Indian pharma organizations like, Shriram Institute for Industrial Research, Morepen Laboratories, Dr. Reddy’s Laboratories, Ranbaxy Laboratories, Sun Pharmaceuticals and Glenmark Pharmaceuticals holding strategic leadership positions.
>> see the abstract about Digital Pharma Laboratory - challenge | opportunity | way forward
 Presenter Rik Pepermans, consultant (ex- IT Innovation Lead, R&D IT Evangelist at Unilever)
Presenter Rik Pepermans, consultant (ex- IT Innovation Lead, R&D IT Evangelist at Unilever)
#Research #Automation #Standardisation

 Presenter: Albert Almajano, Chief Information officer at Grupo Indukern
Presenter: Albert Almajano, Chief Information officer at Grupo Indukern
#Digital Strategy #Lockdown #IT Business partners #Change Management
Albert is an experienced Area Director with a demonstrated history of working in the Pharmaceutical Industry. Skilled in IT/DigitalStrategy, Business Strategy, Negotiation, Digital Transformation, and Team leadership. Strong information technology professional graduated from ESADE Business & Law School and IESE.
Albert is also a teacher at La Salle Campus Barcelona, contributing to the Masters of Information Technology Management and eHealth.
>> see abstract about Roadmap to Digitalisation with Success

*agenda subject to changes

