- #PLA2020, 8th Edition, Central Theme -
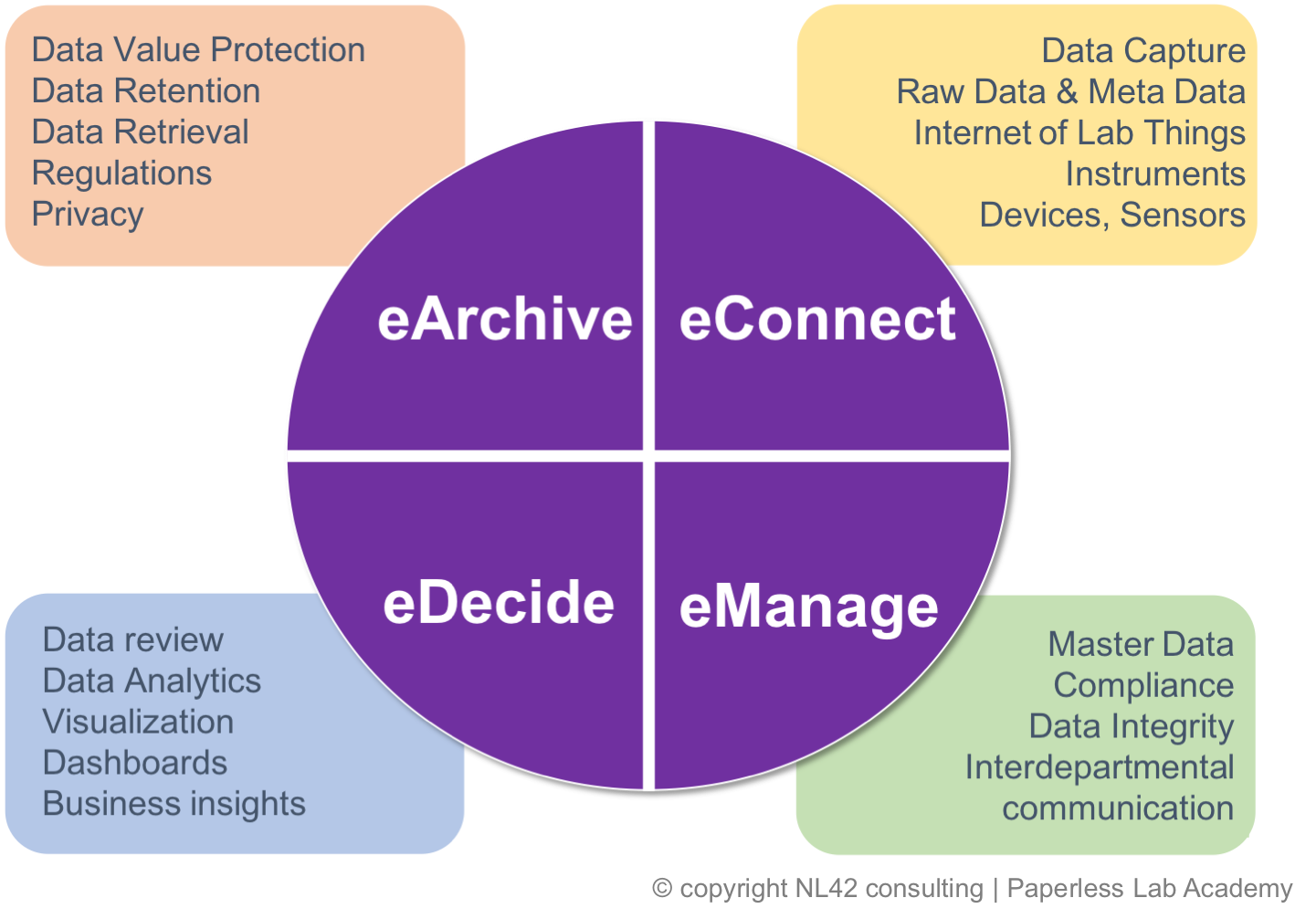
Master your eData Lifecycle
Foster Digital Revolution in your Lab
 The Paperless Lab Academy is the ideal learning platform for all companies that own a laboratory, involved in running, consolidating, integrating or simplifying laboratory data management processes.
The Paperless Lab Academy is the ideal learning platform for all companies that own a laboratory, involved in running, consolidating, integrating or simplifying laboratory data management processes.
Everything you always wanted to know about Lab Information Management
Once again, we´re setting the stage for discussions on strategies and implementation of 21st-century technologies in your laboratory.
We work on an agile combination of future horizon views - already running in some industries - and tangible real cases on how to solve today’s concerns.
WHAT WILL YOU LEARN
- About the future of the laboratory within the industry 4.0
- Business decisions and companies’ processes supported by scientific data management
- The relevance of a Master Data strategy for mastering your data
- The reality of the #IoLT, Internet of Lab Things and #Ioi, Integration of instruments
- How to apply the FAIR principles and secure your data
- Why archiving is the beginning and not the end of a solid #eDataLifeCycle
- Practical sessions on Audit trails, data integrity, and new Compliance guides
- Demystification of the Cloud-based solutions
- How to successfully implement laboratory system solutions
WHO SHOULD ATTEND
Industries that significantly benefit from the Paperless Lab Academy congress include: Pharmaceutical, Lab Services, Biotech, Food & Beverage, Consumer Goods, Cosmetic, Chemical, Oil & Gas
People visiting us every year range from Laboratory Directors and Managers, Operational Excellence, IT Business Partners, QC, and QA Management, Research departments, and Manufacturing
 Mark Newton comes over to Europe and offers a unique opportunity to get introduced to best practices
Mark Newton comes over to Europe and offers a unique opportunity to get introduced to best practices
[due to change of date - Training timing is still to be confirmed ]
Attendees will know better about the critical issues to examine prior to purchase, how to use vendor information, both about quality of the vendor’s process and the software’s design. Participants will also know where they should perform testing, based on criticality of requirements, where to use vendor’s testing, and what controls will need to be developed during validation to deploy the system in a regulated environment.
#succesfulimplementation #computersystemvalidation



 Presenting: Isabel Muñoz-Willery Ph.D. & Roberto Castelnovo, owners & organizers at NL42 Consulting | Paperless Lab Academy
Presenting: Isabel Muñoz-Willery Ph.D. & Roberto Castelnovo, owners & organizers at NL42 Consulting | Paperless Lab Academy
Isabel and Roberto are entrepreneurial persons involved in paperless laboratory processes projects. Coming from international managerial roles with strong domain knowledge in laboratory processes, laboratory automation, laboratory informatics, and project management, they launched their personal project, NL42 consulting, by 2012. Soon their own customers were asking for better visibility of the industry solutions and learn about the latest technological trends and best-recommended methodologies.
Along the time, the Paperless Lab Academy has become the platform where to find the answers.
 Dr. Lloyd F. Colegrove, Director of Data Services at Dow Chemical
Dr. Lloyd F. Colegrove, Director of Data Services at Dow Chemical
Lloyd F. Colegrove is also the Director of Fundamental Problem Solving within Manufacturing and Engineering and the Analytics Platform Director for Dow’s Manufacturing and Engineering’s Industry 4.0 program. Lloyd’s background is in Chemical Physics where he obtained a B.S. and Ph.D. from Texas A&M University. He spent 7 years in Polymer Research in Dow (R&D and TS&D) before moving into Manufacturing in an Analytical Improvement role and then a Quality Leader for five Dow businesses before moving to help establish a new capability called Fundamental Problem Solving, where top chemists and engineers work on complex, often multi-effect, plant problems.
>> see more about Winning the battle of culture change in manufacturing analytics


Eric Gressier, IT Direction for Research & Innovation at Danone
Graduated of the EM Lyon Business School with a specialization in information systems and controlling, Eric Gressier began his career at Gattefossé GmbH in 2002 before joining Evian and then the Danone Group in France and in the Netherlands. After 6 years of experience in information systems as products owner (SAP, SFA, BI tools) and business analyst for the sales department, Eric joined the IT teams to successively manage the support and third-party application maintenance of the French & corporate digital enterprise department and then lead the worldwide set-up & deployment of HR systems such as Cornerstone & SuccessFactors. Eric took the responsibility of IT Director for Research & Innovation in 2016 on a global scope (Paris, Utrecht, Singapore) to enable the digital transformation.
 Clara Kao, IT team Leader for Research & Innovation at Danone
Clara Kao, IT team Leader for Research & Innovation at Danone
Clara has a background in Microbiology, Food Sciences and Information System. She started her career within Danone in 2000 after her PhD in the “Cultures and Fermentation” R&D department. She then moved to Quality and Food Safety where she has been working on biological risks assessment. During these years, she has always been an information systems activist, implementing solutions aimed at optimizing the management of knowledge. After several roles of both business project leader and change manager on IS projects, she decided in 2013 to devote herself to IS.
She is now managing the IS team delivering service for Danone R&D, having in her scope amongst others LIMS, ELN…. She is also acting to leverage the value of new technologies for the R&D laboratories and is a strong promotor of a sustainable and responsible digitalisation
>> see more about Danone R&I Lab Digitalisation: beyond the hype, from injunction to user reality

 Toni Manzano, Chief Science Officer, R&D Director and Co-founder at Bigfinite
Toni Manzano, Chief Science Officer, R&D Director and Co-founder at Bigfinite
Toni is the co-founder and CSO of Bigfinite, a cloud company that provides big data and AI SaaS platform for the Biotech and Pharma industry. He is also member of the scientific committee of the PDA Europe, Lead of the AI in Operations team for the AI Xavier University and teaches AI subjects at the University (UAB), member of the Science Experts in the Spanish Parliament on big data and artificial intelligence topic. He has written numerous articles in the Pharma field and holds a dozen international patents related to the encryption, transmission, storage and processing of large volumes of data for regulated environments in the cloud.
Toni is Physicist, Master in Information and Knowledge Society and post graduated in quality systems for manufacturing and research pharmaceutical processes.
>> see more about Data Centricity: What is behind the AI implementation in Pharma

 Nicolas Argento, ELN/LIMS services manager at École Polytechnique Fédérale de Lausanne, EPFL
Nicolas Argento, ELN/LIMS services manager at École Polytechnique Fédérale de Lausanne, EPFL
For the last 5 years, Nicolas has been transforming an ELN/LIMS project into a core service serving the research laboratories and technology platforms in an academic life sciences research institute. Focused on the added value for scientists and laboratory staff, he coordinated 55 LIMS implementations in a wide variety of laboratories. His team of 4 engineers ensure tailor made deployments, the maintenance and evolution of an in-house infrastructure. IT engineer by training, his previous experience was in IT project management in the banking industries. He decided to embrace an innovative training to specialised in IT for healthcare. Acting as a lead link between laboratories and IT departments, his expertise covers biology R&D, healthcare and telemedicine information systems as well as IT project and services management.
>> see more about Institutional ELN and LIMS deployment in Academic research


Francesca Lega, Head of Quality Assurance at the Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe)
Francesca graduated in Chemistry at the University of Padua, Italy. She specialized in “Food Chemistry and Technologies” at the University of Bologna, Italy. From 1997 to 2005 she worked as a chemist for a private company in the field of water and beverage analysis. She has been a Chemistry manager at the Anabolic Laboratory at IZSVe since 2005 and from 2010 onwards she has been the Head of Quality Assurance of the Chemistry Department.
 Federica Gallocchio, senior scientist at the Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe)
Federica Gallocchio, senior scientist at the Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe)Federica graduated in Chemistry andPharmaceutical Techniques and obtained her PhD in Pharmaceutical Science at Padua University (Italy).
She completed the specialization diploma in Food Science and Nutrition at the University of Padua in 2012. She has been working at the IZSVe since 2007, where she has been dealing with the development and validation of analytical methods for the detection of organic and inorganic contaminants in food and feed.

 Andreas Steinle, Manager Digital Solution at Roche Diagnostics GmbH
Andreas Steinle, Manager Digital Solution at Roche Diagnostics GmbH
Andreas Steinle is accountable for digital transformation in the analytic department of Roche Pharmaceuticals Technical Development Europa since early 2018.
Between 2005 to 2017, Andreas was head of the group “Analytical Systems” with the responsibility for support of validation of analytical systems and laboratory equipment (pH Meter, HPLC, LIMS, Archiving, etc.) in Roche Pharma Biotech Production in Penzberg.
In addition in this role he he was also responsible for the integration of laboratory equipments into enterprise systems and the representation to health authorities.
>> see more about Unlock your analytical instrument data for data science and more

 Eric De Maesschalck, Head Laboratory Digitalization, Corporate Processes and Operations at UCB Pharma S.A.
Eric De Maesschalck, Head Laboratory Digitalization, Corporate Processes and Operations at UCB Pharma S.A.
Eric graduated in Clinical Chemistry and Medical Biology. He has spent 32 years in highly regulated pharmaceutical companies: GSK, Pfizer, Phibro Animal Health and UCB BioPharma. Eric has 27 years of experience in computerized systems qualification and validation including IT infrastructure (GMP, GCP, GLP, GPvP). Eric’s position at UCB Pharma is Head Laboratory Digitalization, Corporate Processes and Operations where, among other things, he is defining and following the digital transformation path of laboratory analytical and data processes in partnership with laboratory management and IT
see more about Business Case : Entering the Digital Era for Environmental Monitoring Data Processing

 Daniel Juchli, Head of Lab & Research IT at wega Informatik AG, Switzerland and Chief Technical Officer at the SiLA Consortium
Daniel Juchli, Head of Lab & Research IT at wega Informatik AG, Switzerland and Chief Technical Officer at the SiLA Consortium
Daniel's professional background is based on a dual qualification, as a chemist and as an IT professional. He has over 20 years of professional experience in both disciplines. Daniel speaks both the language of scientists and IT experts and can translate between the participants in project teams. His life science knowledge enables him to bridge the gap between the needs of users and the technical possibilities of IT systems.
>> see more about Digital Lab in a Nutshell

 Mark Newton, Principal at Heartland QA
Mark Newton, Principal at Heartland QA
Mark is an independent consultant who specialises in data integrity, laboratory informatics, computer systems validation, and Quality. He has 35 years of experience in the pharmaceutical industry in QC Labs, computer systems validation and lab informatics at Eli Lilly. Mark co-lead Eli Lilly’s data integrity remediation program for QC Labs worldwide in 2012, consulted and audited several Lilly sites preparing for data-integrity focused inspections.
Mark is a co-leader for the GAMP Data Integrity Special Interest Group and Chair of ISPE Global Documents Committee.
Co-author of "Harmonizing USP <1058> and GAMP for Analytical Instrument Qualification" Schuessler, Newton, Smith, Burgess, McDowall. Pharm. Engineering, Jan/Feb 2014
Co-editor of the GAMP Good Practice Guide "A Risk-Based Approach to Compliant Computerized Laboratory Systems" Nov. 2012.
*agenda subject to changes



