Discover the PLA2023 USA Program
 The Ecosystem for Digital Transformation is All Around Us!
The Ecosystem for Digital Transformation is All Around Us!
Pat Pijanowski, former Managing Director at Accenture Scientific Informatics Services
The Cloud, Edge Computing, 5G Networks, Low-Code/No-Code BPM solutions, Artificial Intelligence & Machine Learning, Augmented and Extended Reality, Broadband communications around the world…. The ecosystem for digital transformation is all around us! One only needs to look at the many ways in which our everyday lives have been transformed over the past couple decades to see the promise of the tools available to us in today’s world. And yet, so many organizations have struggled to leverage these same digital enablers within the laboratory environment. Why is that? And what must we do?
Successfully bringing Digital Transformation to the Lab requires us all to think about things a bit differently than we have in the past. Successful Digital Transformation requires us to adopt an “Ecosystem Mindset” instead of an “Application Mindset.” And the good news is……. It’s Happening!!!
 There is No Such Thing as “Just” a Digital Transformation
There is No Such Thing as “Just” a Digital Transformation
John F. Conway, Chef Visioneer Officer at 20/15 Visioneers
Culture Change enables strategy adoption and the ability to adopt new strategies such as a Scientific Data/Process and Decision Strategy and make them actionable. What also transforms is the science and technology as well as all their processes and very importantly the “unknown” art of the possible! This talk will highlight current use cases and lessons learned.
 Fundamental Problem Solving, a simple but transformative discipline
Fundamental Problem Solving, a simple but transformative discipline
Lloyd Colegrove, former Director of Data Service at Dow Chemicals
Digital transformation and culture change go hand-in-hand and are critical to taking advantage of what new digital technologies have to offer, but in your operational environment have you ever considered how your newly transformed and digitally enabled workforce will successfully perform to solve operational problems and improve operational performance?
"Fundamental Problem Solving" is a discipline formalized around the old elementary school science fair concept of hypothesis and testing, combined with the complexities of "adult" technological issues around problem identification and solving.
Three questions, which your elementary school science teacher did NOT teach you, form the basis for powerful industrial problem solving of operational issues that mysteriously recur even after you think you have put them to rest, or identifying and mitigating so-called "multi-effect" problems - problems that do not have one clear or obvious root cause. Serendipitously, the precepts of fundamental problem solving can be applied to all aspects of life including but not limited to - career development, family vacation debates, and even where you and your partner may go for dinner tonight!
Followed by a Panel discussion with the keynote speakers about how to adopt a digital mindset.
 Leveraging cross-business synergies as pathway to digitalisation
Leveraging cross-business synergies as pathway to digitalisation
Karen Smalara, Senior Principal Software Engineer at Boehringer Ingelheim
The case of the MyLIMS program currently under way at Boehringer Ingelheim will be presented as a pragmatic approach to address the digitalisation of two apparently diverse businesses such as Animal Health and Biopharma with one holistic template-driven solution which brings significant benefits in terms of simplification of the application landscape, and is the foundation for ongoing and future integration with ERP, MES, LES , CDS and paperless mobile-based sample handling.
 Advanced digitalization of QC-Micro Processes: How users and organizations can go beyond just paper on glass
Advanced digitalization of QC-Micro Processes: How users and organizations can go beyond just paper on glass
Cortney Moses, Quality Control Manager @ GENIXUS
Utilizing real-world examples, Cortney Moses will showcase the transformative impact achieve through strategic digital integration at a Cell & Gene Therapy organization. Attendees will gain a comprehensive understanding of
- The critical role of a robust digital strategy
- Elevated capabilities achieved through the innovative & proactive application of the MODA® platform
- How digital transformation can enable companies to maintain a competitive edge.
 Managing Change in Multiple Laboratories: LIMS Deployments and the Benefits of Standardization
Managing Change in Multiple Laboratories: LIMS Deployments and the Benefits of Standardization
Geoffrey Trullinger , IT Global Lab Automation Software Operations Manager @ Catalent Pharma Solutions
Effective change management is crucial when deploying a Laboratory Information Management System (LIMS) to multiple laboratories. This presentation will explore key strategies and best practices for navigating the complex process of LIMS deployments, including determining lab readiness, identifying opportunities for standardization, and maximizing user adoption. Join us as we delve into the essentials of change management to drive your successful LIMS deployment journey.
 Balancing Business Needs against Compliance Gaps
Balancing Business Needs against Compliance Gaps
Mark Newton, owner at Heartland QA. Former Associate Senior Consultant QA at Eli Lilly
It is important that compliance remains a function that is judged on how it supports the business. However, it is important that the business understands how important quality requirements, and therefore compliance, are to the success of the business itself. Mark will take us in his presentation from his 30+ years at Eli Lilly as an Associate Quality Assurance Senior Consultant and his great contribution as co-editor of several GAMP guides.
 Data Integrity Maturity Model to assess Risks
Data Integrity Maturity Model to assess Risks
Chinmoy Roy, CSV and Data Integrity SME, Life Science Digital Transformation Consultant at Valgenesis
Chinmoy will develop further about the approach to data integrity risk management using data integrity maturity model and process mapping. He will also bring a case study about conducting DI risk assessment in a QC laboratory.
Chinmoy is a biopharmaceutical consultant with over 40 years of experience in CSV, Data Integrity, 21 CFR Part 11, Annex 11 and manufacturing process automation. His presentations blend his field experience to highlight the intricacies of implementing regulations.
 Human Factor: the Data Integrity Wild Card
Human Factor: the Data Integrity Wild Card
George Bernstein, Ph.D., President of MAI Consulting
In his presentation, George will touch upon the opportunities and human factors that lead to data integrity breeches, and strategies to further mitigate that risk. We have engineering and software solutions to reduce opportunities for data integrity breech, but these are applicable for some but not all areas of exposure. Use of legacy equipment, logbooks, and manual entry into batch records requires training and QA oversight to minimize data integrity risk. Unfortunately, training is often ineffective, and QA staffing may be in short supply. George has a Ph.D. in Chemical Engineering and over 30 years of experience in pharmaceutical manufacturing, laboratory operations, data integrity, effective training, and quality risk management.
Followed by a the panel discussion about how to leverage Quality Culture to Drive Quality Production
>> read more about :PLA2023USA: Quality Production relies on Fundamental Quality Culture
 Labs of Today - The Real-World Adoption of Digital
Labs of Today - The Real-World Adoption of Digital
Don Rainville, Global Innovation Hub Lead @ Accenture Scientific Informatics Services (ASIS)
Organizations have spent 20 years sharpening their saw when it comes to deploying foundational digital lab capabilities. Now more than ever, with the dominance of cloud and confidence in more advanced technologies, attention is turning towards the truly transformational and aspirational ways lab scientists can work in digital environments. These capabilities are generating enormous value and unleashing scientific ingenuity across industries.
Followed by Panel Discussion about how to leverage digital experiences to drive new strategies
 Driving Digital Transformation for R&D agility and product innovation
Driving Digital Transformation for R&D agility and product innovation
Kumar Subramanyan, Director - Global Modelling & Analytics, Research & Development at Unilever
R&D is in the midst of a large-scale digital transformation with a view to innovating boldly for “people and planet”, while meeting the consumer needs of today and tomorrow. Access to large amounts of data and use of high-performance computing and advanced data analytics, has enabled “in-silico” innovation and virtual simulation at scale – massively shortening the time it takes to ideate and design compelling product innovations.
To highlight how this digital transformation has been implemented, examples will be showcased on how efficient data capture systems can enable value creation through analytics including AI/ML approaches in the areas of consumer insight, formulation design and product evaluation.
 There should be nothing Artificial about Intelligence
There should be nothing Artificial about Intelligence
Lloyd Colegrove, former Director of Data Service at Dow Chemicals
The journey towards a major chemical company’s vision for the artful use of large structured and unstructured data sets in real-time plant operation and problem solving is a story of technology, salesmanship, and the hard work of convincing a company in love with Excel spreadsheets, and problem solving after-the-fact, that significant value lies in already collected and often under-utilized plant data sets. Part of the story includes the application of artificial intelligence, but AI is not the solution, it is a solution. Through the use of volunteer audience participation and a few artful stories, this talk will discuss real-time analytics success stories that are actually simpler than “AI” infused solutions, discuss where AI fits in the chemical industry, and highlight some experiences from 30 years of engagement in chemical manufacturing that can bring tremendous bottom-line value to your chemical operations.
Followed by a Panel Discussion about data-driven decision approach for successful DT acceptance
 Transforming biomanufacturing: breaking through today’s digital technology tropes
Transforming biomanufacturing: breaking through today’s digital technology tropes
Luke Guerrero, Chief Operating Officer @ QbD Vision
It’s finally here: pharma and biotech are seeing digital transformation projects and activities pick up across the board. The problem has shifted from priority and budget to a discussion on focus and starting points. As organizations scale their software usage, master data management becomes a conversation, especially when the lab meets enterprise. But is it the right discussion to have first? What are the blockers? This talk summarizes the current state and suggests pathways for organizations that think differently and want to learn from the successes in biotech and other industries.
 How communication and data standards enable the automation and data management lifecycle
How communication and data standards enable the automation and data management lifecycle
Patrick Courtney, Board of Director @SiLA Consortium
Followed by Panel Discussion about how to master your data life cycle
Evaluation, Integration and Value of a Fully Connected Lab
Lab instruments and enterprise systems that solve specific needs reside in silos within a manufacturing site.
Connecting these devices and systems provides the ability to capture for better, more efficient decisions, and will error proof the workflow by removing data transcription. The how, why, when and for whom should organizations connect devices and systems will determine how efficiently and effectively organizations can derive value from the data they generate, and ultimately, error proof workflows and processes.
A thoughtfully designed, connected lab, and connected data add business value by allowing sites to increase capacity.
This session will discuss the value of defining integrations, the technical aspects of having devices and systems exchange data, setting up validation protocols to manage data integrity concerns, and the process improvements that will ultimately be realized.
AI in the Lab: Strategies to adopt a Data Driven Future
In this workshop, the speaker explores the potential of ontologies to address the big data challenges faced in the life sciences industry.
By making data FAIR: Findable, Accessible, Interoperable, and Reusable, ontologies help facilitate efficient data management, reduce search time, avoid duplication of work, and enable the utilization of data for AI and machine learning projects.
This allows life science organizations to transform their data management practices and unlock the potential of their data for scientific advancements and positive business outcomes.
Digital Transformation Through Instrument Empowerment
We are all asked to do more with less. For this reason we need to find new and creative ways to automate processes while also reducing the effort required to accomplish daily tasks. By empowering instruments we allow scientists to perform more science and spend less time shuffling between PCs and their samples, capturing and replicating data, and finally ensure all compliance requirements are met. During this session we will analyze a typical laboratory workflow from instrument verification to data being placed in its final location both with software capable of Empowering the Instrument as an interface and without.
>> see more:METTLER TOLEDO workshop #PLA2023USA
6 Use Cases where automation & AI bring lab's scheduling, planning & performance to a next level
During the Second Industrial Revolution, companies knew that electrification was critical to the future of manufacturing but the expected gains were slow to manifest. Early adopters had retained the drive and belt systems that powered their factories and simply replaced the centralized steam engine with an equivalent electrical motor, continuing to mechanically transmit power to machinery. Production only ramped-up after manufacturers changed their mindset and learned how to optimize their processes and infrastructure for the new technology.
In today’s Fourth/Fifth Industrial Revolution, bioscience and pharma companies know that the labs of the future will be fully digitalized but the best approach to implementation is not always clear. Like their forebears, it can be tempting to simply replace the old with the new without also optimizing their processes to take full advantage of the technological opportunities.
In this workshop we will draw on our extensive experience to share some key enablers of optimized laboratory digitalization:
- Examples of how to leverage digitalization to optimize laboratory processes, including:
o Enhancing team organization
o Optimizing decision processes
o Streamlining communication with labstakeholders
- Lessons learned around the impact on analysts and technicians
- Harnessing data to improve laboratory performance (e.g. service level and throughput)
Confirmed PLA2023USA Sponsors: Exhibitors and Workshop owners


From Raw Data to AI
10 live editions where the topics evolved from introducing the concept to presenting use cases demonstrating full implementation. Digital Twin, BlockChain, Cloud, Semantics, FAIR Principles, Standardisation, Internet of Lab Things, Automation, Globalisation, Data Quality, AI.
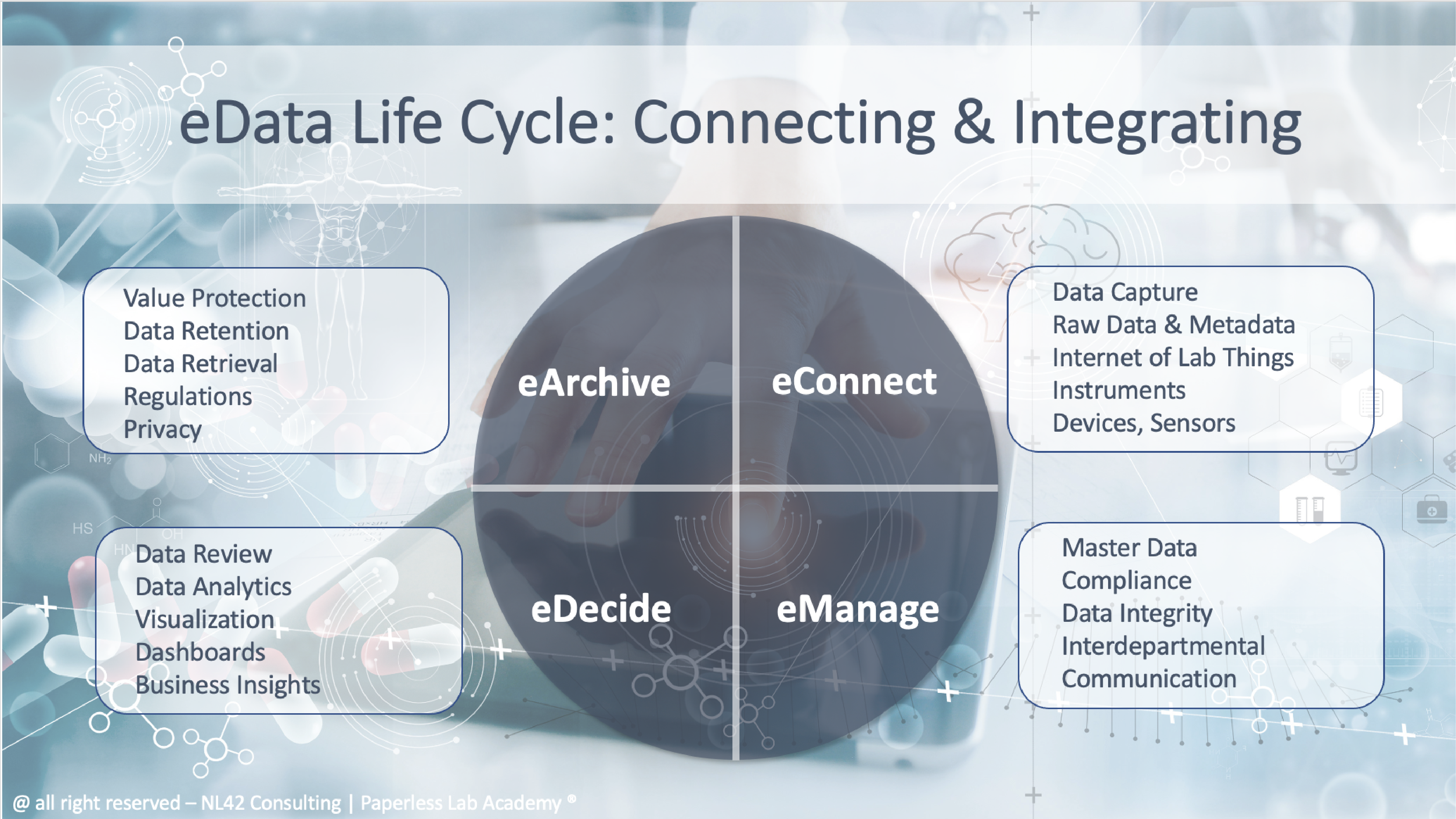
eData Lifecycle Discussions
Digital data have a specific lifecycle from their creation to final storing. At every step those data should be easily reachable and bring the necessary information to facilitate the decision making. Industry compliances also matter here and need to be covered. Yet above all the right management of those data is crucial for its company business.
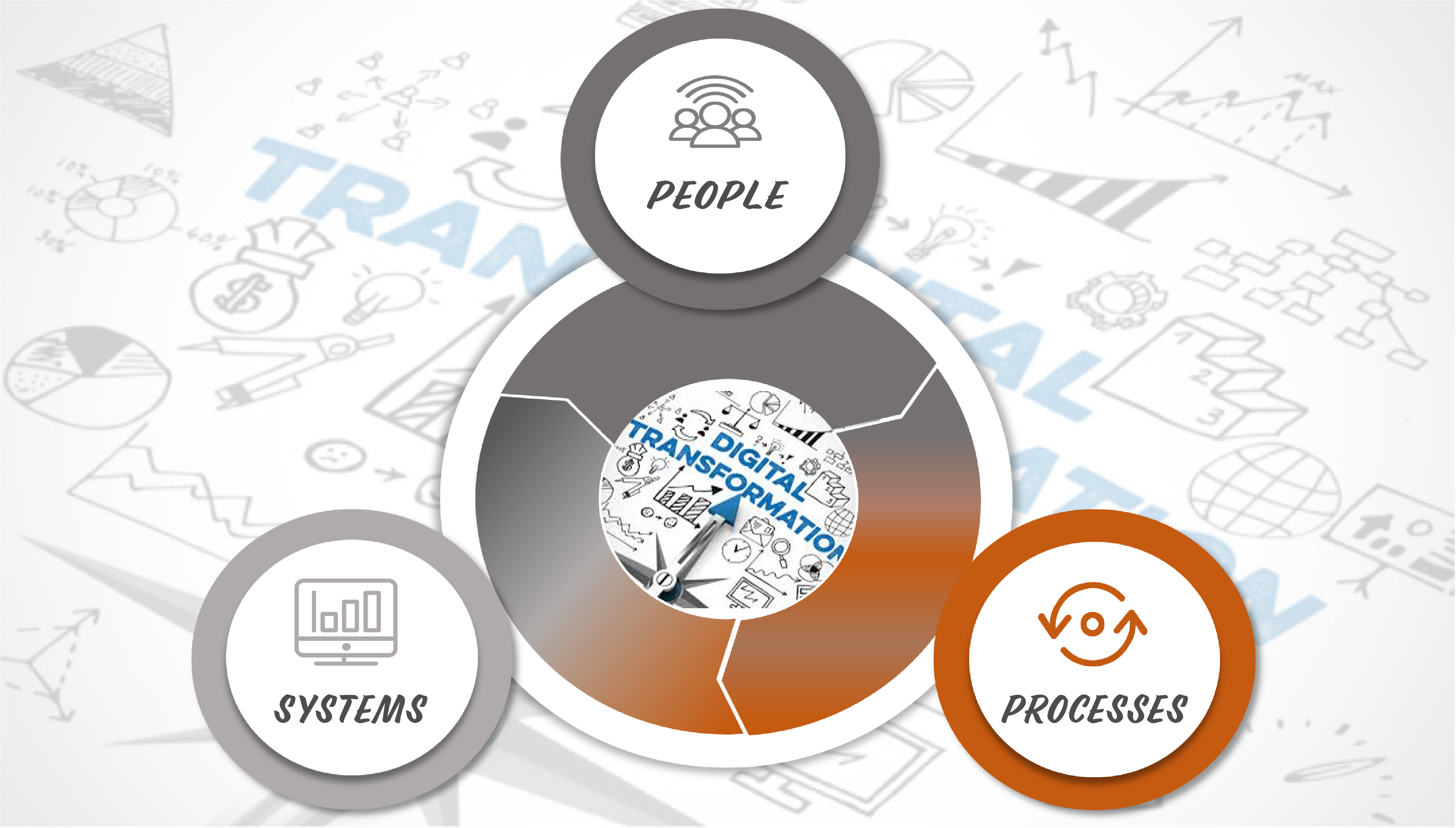
Driving the Change Management
Managing a Digital Transformation project in your company for your laboratory and quality processes is not a flawless project. Beside Your processes and the adequate systems to be selected and implemented, every one that have been through it will always reinforce the point that getting your team on board is crucial. The Human Factor is key for a successful digital transformation project.

Digital Data Management Topics
Topics covered are about Digital Data Management all along the eData lifecycle and all the related matters such as data integrity, data integration, data analytics, data standardisation, structured vs unstructured data, data search, machine learning, artificial intelligence, etc.
PLA2023USA Media Partners

NOT DECIDED YET?
Make sure to remain informed on the latest




