Compliance made easy: How digital management of lab inventory simplifies compliance

Despite digital advances in so many areas of our lives, a surprising number of laboratories are still relying on error-prone and time-consuming paper and spreadsheet-based systems for their laboratory inventory processing. Not only is this affecting their productivity – scientists can spend as much as 25% of their time managing inventory data – but it also causes compliance challenges. Enter LANEXO™ System – an innovative inventory safety and compliance management system designed to help laboratories digitalise their processes to free up valuable time, reduce errors and ensure continual compliance. Imagine always being ready for your next audit.
Real time tracking of consumables
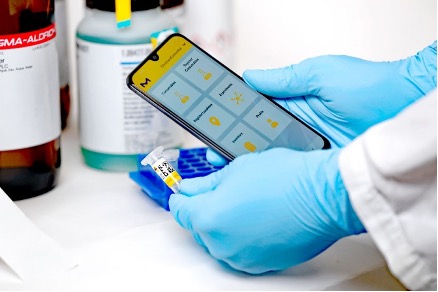 The LANEXO™ System uses digital data capture and smart RFID labels to document consumables and track their use in real time throughout the laboratory. Data can be reviewed via cloud-access for 24/7 convenience – even across sites or when working remotely.
The LANEXO™ System uses digital data capture and smart RFID labels to document consumables and track their use in real time throughout the laboratory. Data can be reviewed via cloud-access for 24/7 convenience – even across sites or when working remotely.
On arrival in the laboratory, consumables are given Smart Seal RFID labels and scanned via a mobile app. The user then scans the RFID Label of the storage location to record and tracks the details in LANEXO™ System. Each time a consumable is used, the label is scanned by a logged in user. This builds up a complete record for each consumable from opening through to disposal – all the information necessary to uphold data integrity, facilitate any experimental troubleshooting and ensure quality.
Avoid cumbersome, error-prone data transcription
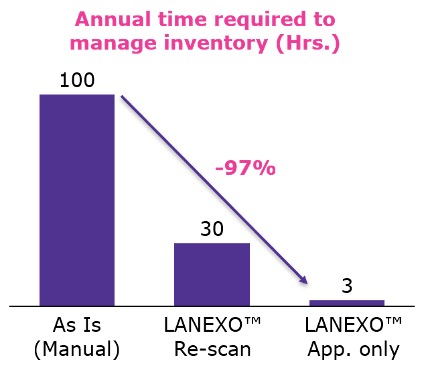 Manual data entry is not only incredibly time-consuming and frustrating, it is inherently error prone. Transcription errors are commonplace, and there is a considerable risk of paper records being lost or mis-filed. LANEXO™ System can reduce the time required to manage inventory by 97% and the time required to check, find and document consumables by 92%. This frees up a considerable amount of time that scientists can use for more valuable tasks increasing laboratory productivity.
Manual data entry is not only incredibly time-consuming and frustrating, it is inherently error prone. Transcription errors are commonplace, and there is a considerable risk of paper records being lost or mis-filed. LANEXO™ System can reduce the time required to manage inventory by 97% and the time required to check, find and document consumables by 92%. This frees up a considerable amount of time that scientists can use for more valuable tasks increasing laboratory productivity.
Prevent use of expired reagents
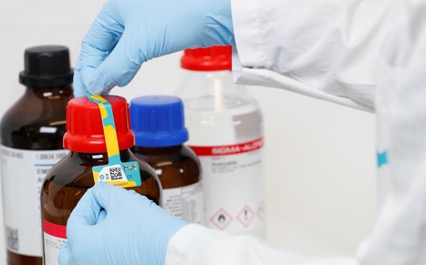 In many laboratories, it could be very easy for scientists to unknowingly use an expired product in their experiments, particularly if expiration dates are based on time since opening. It is also possible that when performing manual stock checks, some expired products are missed. As many as 70% of laboratories report having to manually inspect their records for expired consumables in root-cause analysis – a time-consuming process.
In many laboratories, it could be very easy for scientists to unknowingly use an expired product in their experiments, particularly if expiration dates are based on time since opening. It is also possible that when performing manual stock checks, some expired products are missed. As many as 70% of laboratories report having to manually inspect their records for expired consumables in root-cause analysis – a time-consuming process.
With the LANEXO™ System, a user opening a new reagent will tear the Smart Seal Label, which automatically logs the reagent opening date. LANEXO™ App will then calculate expiration dates based on known shelf life, thereby, eliminating any manual miscalculations or misuse that may impact results. Automated alerts mean that expired products are never used and are instead disposed of appropriately.
Facilitate GxP compliance
Digital data capture combined with the comprehensive digital audit history provided by LANEXO™ System also facilitates GxP compliance. Each reagent can be instantly matched to an audit report; this includes tracking a consumable’s initial registration step, ownership, opening and expiration dates, use to create a mixture or intermediate, use in an experimental workflow and subsequent disposal. With all this data recorded in real time and instantly accessible, this means that laboratories can always be audit ready.
Improve safety and regulatory compliance
Rapid access to appropriate health and safety information is of vital importance to any laboratory. With a database of more than 700,000 Merck consumables and 53 million third-party consumables, LANEXO™ System provides scientists with instant electronic access to safety information, including hazard warnings, and instructions for safe disposal. In addition, automatic notifications alert users to storage incompatibilities (when the rules of segregation, separation and ventilation are breached, for example).
Making compliance less complex
Digital data capture avoids the transcription errors that are commonplace with manual systems. It also enables real time access to that data, from any location. Laboratories using digital inventory management can not only use this to manage stock, but also to build and review a full audit trail showing how each consumable is used in the laboratory aiding troubleshooting and making sure they are always audit ready. The LANEXO™ System includes numerous built-in safeguards that prevent the incorrect use or storage of consumables, reducing errors and ensuring safety. Ultimately, digital inventory management helps to make compliance less complex.
Contact us to learn more about digital inventory management and the LANEXO™ System at LANEXO@merckgroup.com, or visit LanexoSystem.com
Access to the Lanexo Introduction at the Paperless Lab Academy Webinar
 Read More about Lanexo Merck: Digital Consumables Tracking for COVID-Time and Beyond
Read More about Lanexo Merck: Digital Consumables Tracking for COVID-Time and Beyond
Latest Posts
Key Topics of the PLA2024India
PLA2024India, 5th edition, promises a programme full of interactions and discussions. 4 focused sessions and 2 training workshops The main theme of #P
14 May 2024
Press Release: PLA® Conferences to partner with IA-Meetings for its 5th Indian Edition.
The Paperless Lab Academy® (PLA) is a leading conference about digital transformation of laboratory and quality processes. Above all, it is about mas
08 April 2024
The #PLA2024Europe programme aimed to highlight the importance of the human factor in digital transformation with several presentations and panel disc
19 March 2024

