Presentation OSTHUS
An Enterprise-Level Strategy for Reference Master Data Management: Meeting the Challenge to support analytics in the e-Data Lifecycle
Presenter

Name: Eric Little
Title: Chief Data Officer
Abstract
This talk will provide a means to discuss the capture, integration and dissemination of data across large enterprises. We will show how data variety is continuing to grow, meaning new data sources are steadily becoming available for use in analysis. Data veracity is also of importance since a large amount of data is fuzzy (uncertain) in nature. The ability to integrate these various data sources and provide improved capabilities to understand and use it is of increasing importance in today’s pharma climate. We call this Reference Master Data Management (RMDM).
This talk will span an arc of data lifecycle management, beginning with instrument data, moving across to clinical studies, production, regulatory affairs and finally e-archiving (see Fig. 1). I will show how these systems can use a common semantics for modeling of important metadata, which can apply the FAIR principles of Findability, Accessibility, Interoperability and Reusability to a common “semantic hub” that can connect data sources of different varieties across the enterprise. ADF files, for example, use their Data Description layer to provide semantic metadata about file contents. Similarly, semantics can be used to describe clinical trials data, regulatory data, etc., through to archiving, for improved storage and search over long periods of time.
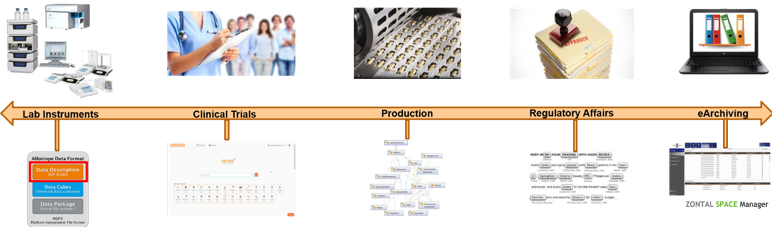
This talk will provide use case examples from work done with pharma customers to show the application of this approach across the various industry silos mentioned. This way attendees will be able to understand both the theory and the practice involved in these techniques and how OSTHUS clients such as Bayer, AstraZeneca and others are using this methodology with success. We will begin by showing the applications in Allotrope to instrument data, then move across the data lifecycle all the way to long-term storage.
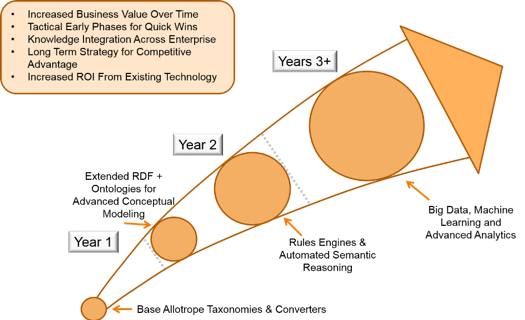
We will also briefly discuss how companies can build a roadmap for this type of application and the kinds of investments and ROI we see from customers who employ this technology approach.