INPHARMATIC WORKSHOP #PLA2020
Learn how to solve legacy instruments Data Integrity gaps with an instrument integration platform
Overview
We will present you an innovative solution able to:
Integrate your laboratory instrumentation resolving their data integrity issues.
Collect instrument measurement detail, enriching it with essential metadata (dateTime, attributability, measurement context…).
Drive the measurement through an approval workflow and review (with Electronical Signature).
Improve reliability reducing human errors.
Generate efficiency. Ioi dramatically reduce no-adding value tasks, providing more time to spend for quality and quantity.
Distribute data captured to LIMS, ELN,.. creating a safe flow data tunnel between the field and the business.
Produce any kind of report based on data acquired managed, according to your needs.
Flexibility, use Ioi wherever you are through Tablet, Smartphone, PC, ...
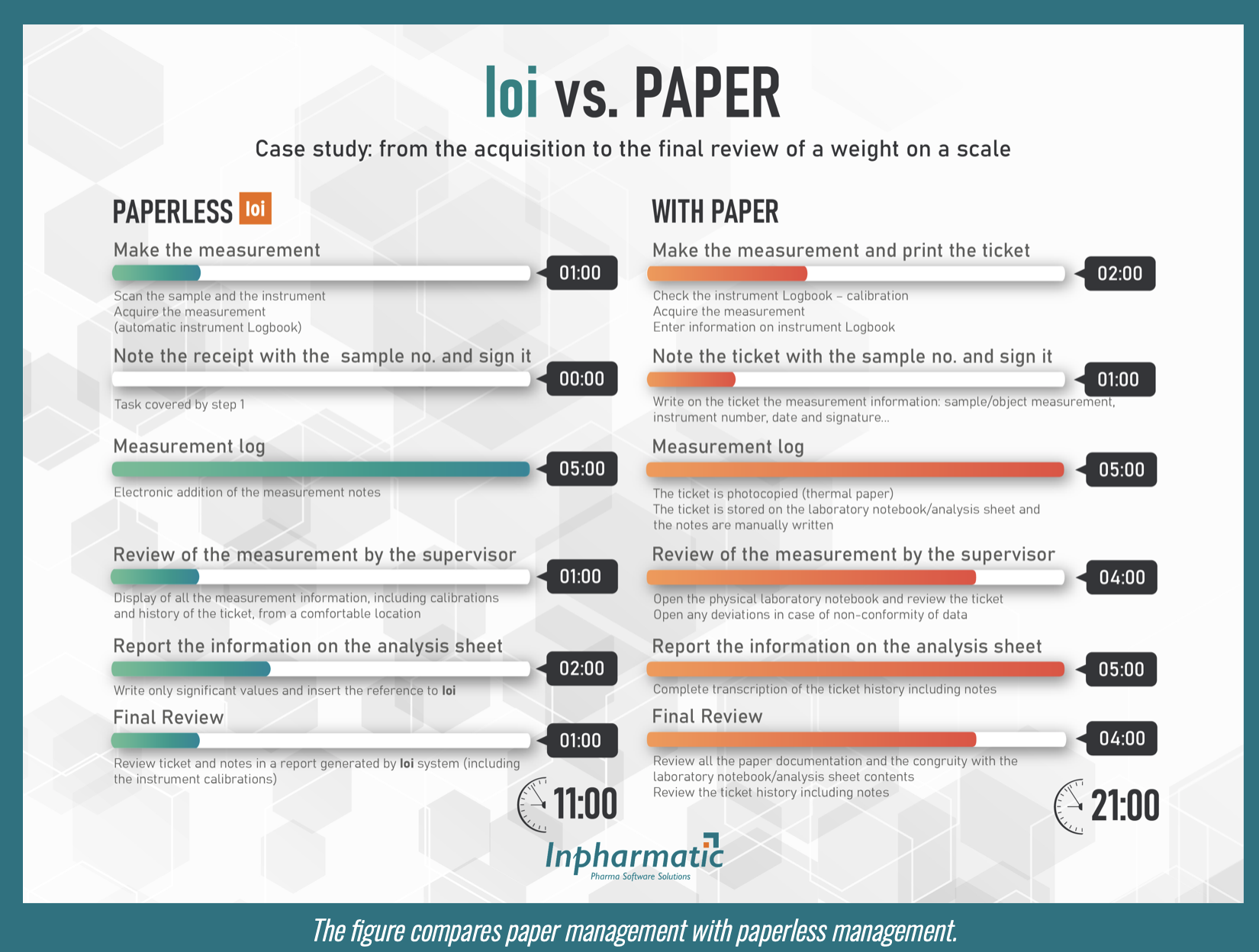
CASE STUDY: IOi VS PAPER
Areas covered in this session
Data Integrity, Simple and Complex instruments integration, Middleware.
Who should benefit
Analyst, supervisor and QA.
Session learnings
Learn how to put in compliance instrumentation with Data Integrity requirements.
Innovative Digitalization Platform for stand-alone Instrumentation.
Learn how to review data in electronic format: improve laboratory/production efficiency.
Presenter
Inpharmatic is a team combining professionals with more than 25 years of experience in the pharmaceutical industry and a new generation of software designers, coordinated by project managers of international experience. The team aims to add the benefits of efficiency and integration to the systems compliance with the requirements of Data Integrity. In this way, investments in Data Integrity become a competitive advantage for the pharmaceutical industries. Inpharmatic is enlarging and structuring its organization in order to support the success of the proposed solution.