Waters Workshop
Durable Data – Securing Your Instrument Data

Presenter: Charlie Wakeham, APAC GxP Compliance Manager at Waters Corporation
![]()
Charlie Wakeham has more than 20 years of industry experience developing and validating computerized systems for regulated production and laboratory environments. As Waters Corporation’s GxP Compliance Manager, she provides practical and pragmatic computerized systems validation assistance and data integrity consultancy to regulated companies implementing Laboratory Informatics in Asia Pacific (APAC) countries.
Some of Charlie’s career highlights are:
- Training APAC regulators including TGA and Medsafe
- Presenting at 2017 PIC/S Inspectors Seminar held in Taiwan
- Contributing to seven GAMP Good Practice Guides
- Awarded ISPE Max Seales Yonker Member of the Year 2019
Charlie has been active within ISPE GAMP since 2001. She was a founder member of GAMP UK, and former Secretary of GAMP Europe. She currently serves as Secretary of GAMP Global Council and as part of the GAMP Data Integrity SIG Leadership Team.
Abstract
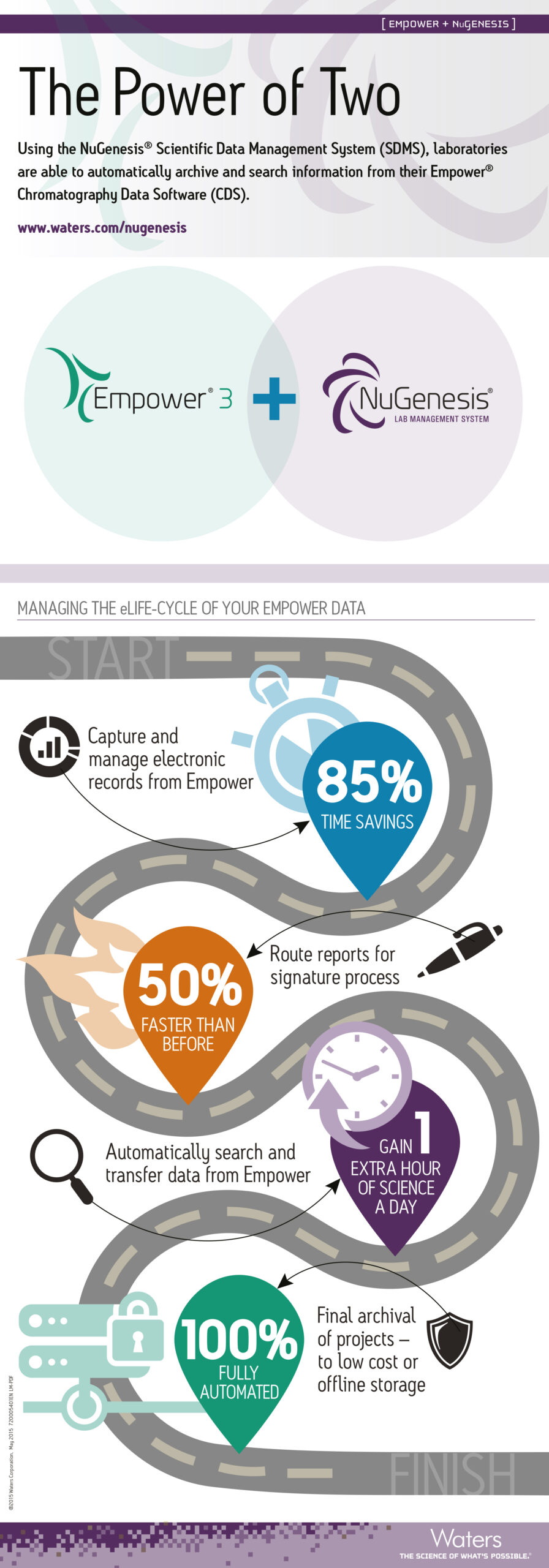
It takes time, money and resources to generate laboratory data, so any loss of data is a direct financial loss to your regulated company as well as a major compliance risk.
This presentation will explain how to simplify record retention using a Scientific Data Management System to capture data from your laboratory instruments. It will discuss key data protection activities needed to protect data from loss or disaster, and how to plan proactively for disasters to significantly reduce their impact on laboratory operations. This presentation aims to help lab managers and quality managers to understand the risks to their regulated data, how to set up safeguards to reduce those risks, and the technologies and solutions that are needed to recover the data if a disaster occurs.