Digital Pharmaceutical Laboratory – Challenges | Opportunities | Way Ahead

What is a digital pharmaceutical laboratory ?
Historically, pharmaceutical laboratories have used paper notebooks/control sheets to keep track of their experimental data, observations and results. The laboratory data and reports are the most important evidence and record of laboratory experiments and testing. A properly maintained raw data, calculations and reports are essential laboratory paper records for any pharmaceutical laboratory.
Enabling electronic access to laboratory data will improve knowledge management capabilities, eliminate the waste of information and enhance efficiencies.
A digital laboratory can sense, communicate, analyse and act on data in an optimal predesigned approach.
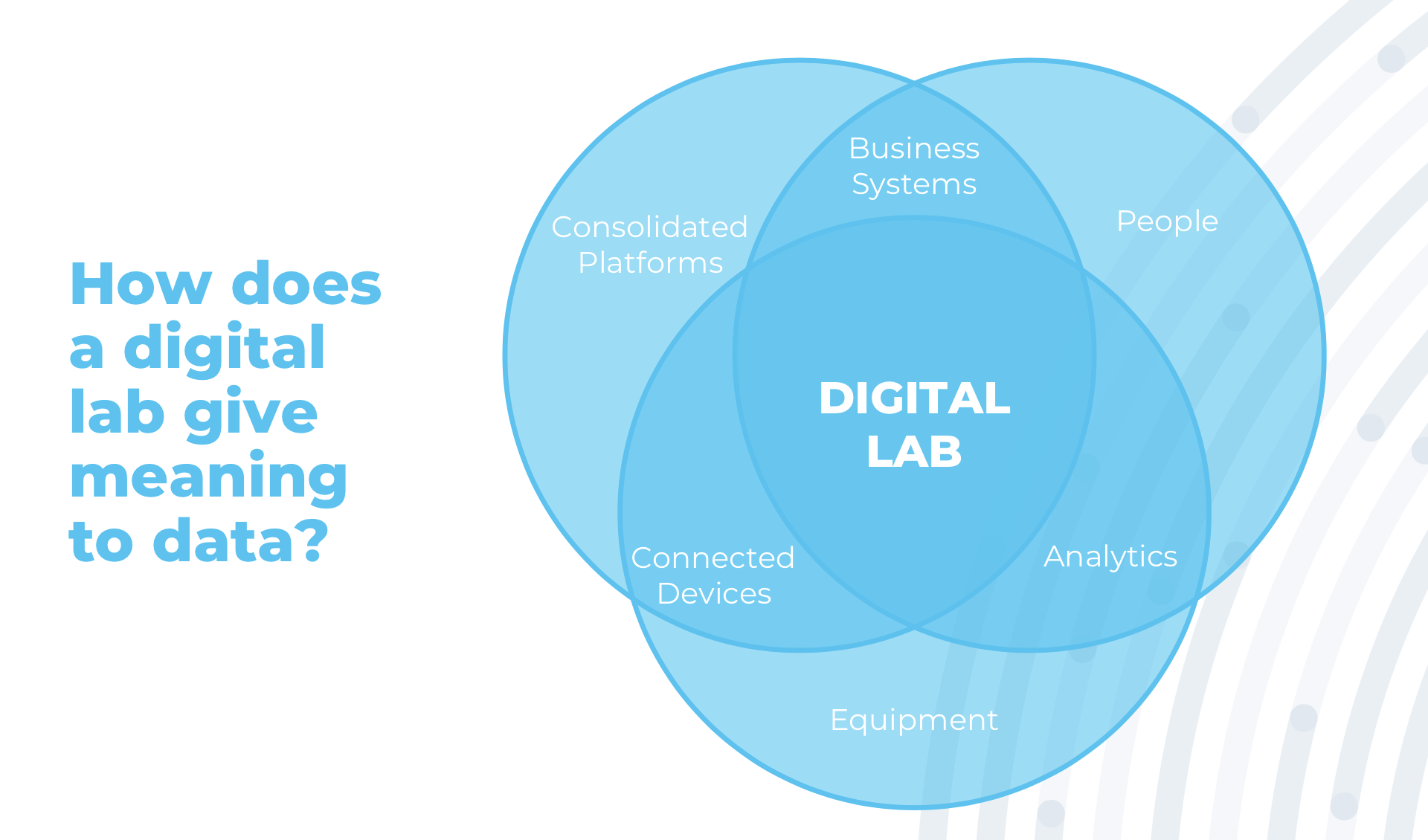 Digital laboratories right at the point of analysis, provide real-time control and automation of testing procedures ensuring procedural execution, automate manual processes, collect instrument data, and perform calculations, limit checking, calibration checking, as well as inventory checking and updating. Digital laboratories also provide electronic documentation and access to test results. These additionally ensure compliance with standard operating procedures and approved methods of testing during the analysis, and protect the integrity of collected data.
Digital laboratories right at the point of analysis, provide real-time control and automation of testing procedures ensuring procedural execution, automate manual processes, collect instrument data, and perform calculations, limit checking, calibration checking, as well as inventory checking and updating. Digital laboratories also provide electronic documentation and access to test results. These additionally ensure compliance with standard operating procedures and approved methods of testing during the analysis, and protect the integrity of collected data.
Digitalized laboratory setups give meaning to data, which enables “digital moments” where decisions are made at the intersection of analytics, business systems and connected devices. These elements are founded on the combined support of people, laboratory equipment, consolidated platforms, and consumables.
Why going got digitalised laboratory?
As per a recent market research, the global market for digital laboratory is valued at about USD 300 million. Majority share of this market currently belongs to life sciences industry alone.
As per a research done through pharma survey, 91.2% respondents claimed to be involved in at least the planning phase of some sort of digitalization adoption and usage (i.e. LIMS, SDMS and Work Flow management).
There are significantly encouraging trends noted, for making shifts towards digital laboratories. Digital labs will add intelligence to existing electronic data with ‘Data Analysis’ algorithms. They will allow mobility for quick and easy access to information, on the go. They will also propagate simulation and prediction tools to reduce duplication and provide opportunities to integrate incongruent systems using a single platform/ single software, with capabilities of integrated information solutions through a single click access.
Regulators are expecting more and more electronically enabled laboratories, with reduced manual interventions. Digital laboratories require to comply with 21 CFR Part 11 guidelines of the Food and Drug Administration (FDA) with respect to digital signatures, electronic records management, and software quality systems management – such as data storage, export, and printing.
For companies having multiple laboratories, disparate instruments, and varied work-flows; data management to the required standards is challenging. As a result, companies are increasingly implementing various digital solutions that take full account of the specific requirements of pharmaceutical manufacturing, and support both quality assurance and continual process improvement.
Industry perspective
The need for digitalization in the pharmaceutical laboratories is continuously evolving. Definitions of digitalization are constantly building. Each geography has a different perspective of and understanding about digitalization. Many believe LIMS is the end of digitalization for laboratories. Many are still searching for a fully integrated solution that meets industry requirements and expectations.
Electronic labs of today lack agility and accuracy. They have various short comings: –
- Today, laboratory equipment feed data to systems where analytics filters it for relevancy; however, humans still interpret most of the results.
- Automation is often built into laboratory informatics, but the systems are not dynamic or flexible. For example, if the performance of an instrument has been diminishing, the experimental deviations are typically unnoticed until the results go ‘out of trend’.
- Historical relationship between an operator and a process is difficult to capture. Mistakes, oversights and challenges are routine even in a paperless or electronic environment, despite using laboratory systems with fail-safe capabilities.
While these systems are electronic in nature, legacy architecture and infrastructure prohibit them from adopting true digital capabilities of the systems.
So, laboratory managers having certain aspirations/ expectations from a digital laboratory:
- What if the sample could itself “suggest” the method of testing most compatible to itself?
- What if a system could predict a preventative measure based on historical data?
- What if a rule based learning engine could suggest improvements based on an instrument’s performance?
- What if the systems could predict availability of various instruments based on their workflow?
Companies are still struggling to find solutions to problems and questions that arise in the process of converting paper laboratories to digital laboratories. As per the research done through pharma survey, it was found that: –
- 41.9% firms saw integration with other laboratory systems as the most challenging area
- 25.8% named the continued integration of applications with existing lab infrastructure as a key obstacle in the long term
- More than three-quarters of firms questioned in the survey described interoperability as a “concern” going forward.
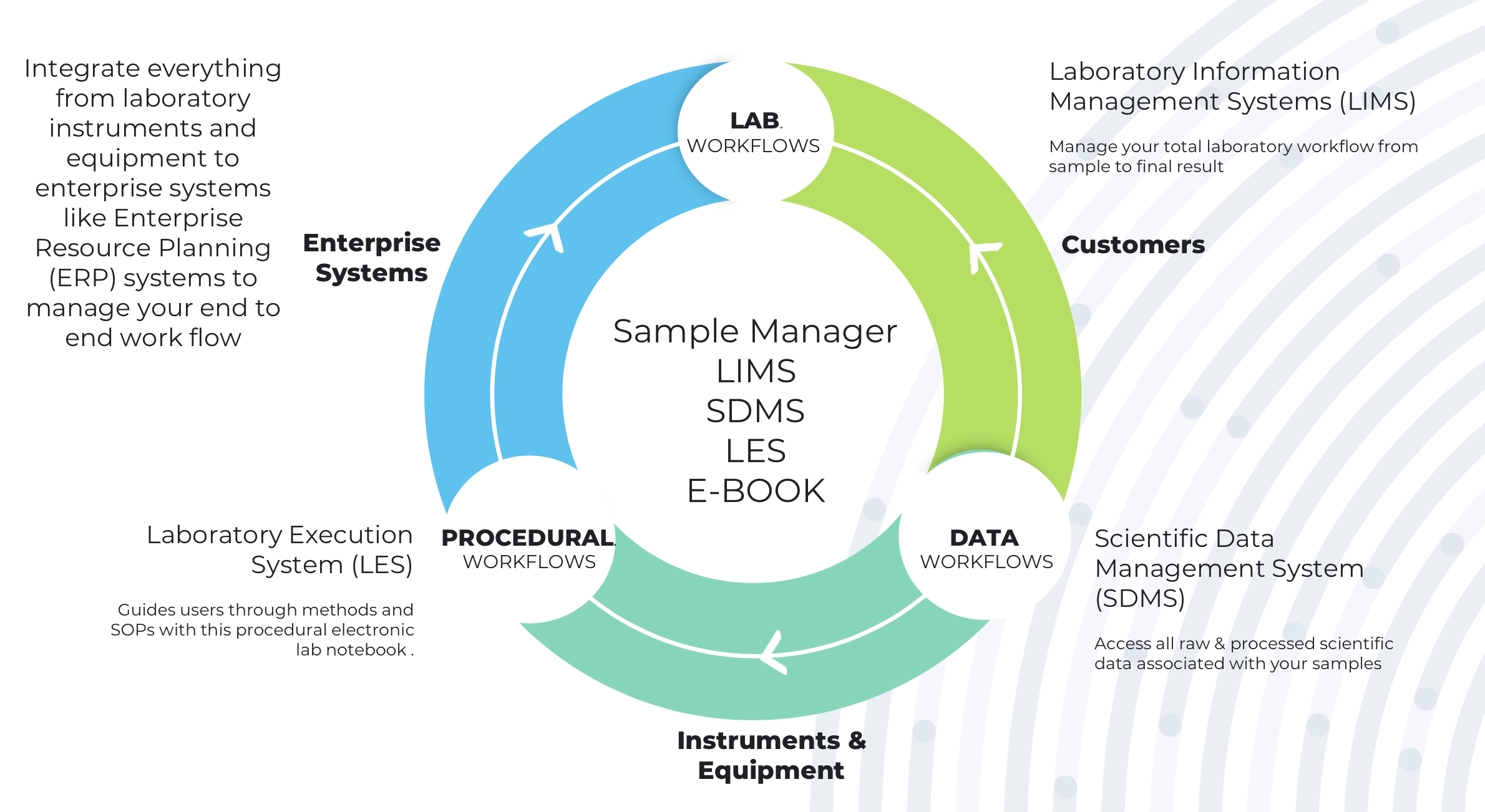
While for many companies, paper-based approaches are already a thing of the past, their replacements may be a range of digital systems, which increasingly require greater integration. Laboratory functions are often managed using a variety of tools. These can include Lab Execution Systems (LES) that drive laboratory procedures, and Scientific Data Management Systems (SDMS) to integrate instruments across the facility and centralize data capture. Laboratory Information Management System (LIMS) that is designed for pharmaceutical manufacturing and QA/QC laboratories, bring together every element of the laboratory. However, there is growing demand for a more unified approach to laboratory and data management.
With LIMS, SDMS and LES software, organizations can integrate everything from laboratory instruments and equipment to enterprise systems. This unified approach should be scalable and flexible, that can be implemented in more than one site and tailored to suit different project requirements. This is exhibited & explained in the picture below.
Key Challenges when implementing an integrated solution framework
There a few key challenges faced when implementing an integrated solution framework in pharmaceutical laboratories:
- Integrated solutions are expensive: Cost incurred is based on the number of applications and data types including instruments, integration as well as number of licensees required
- Varying implementation time: Implementation time varies depending on the scope of the project (number of laboratories, plants, geography, instruments, applications and so on)
- Integration with instruments: Integration depends on number of instruments needed and their compatibility with the integration solution. Each laboratory has multiple instruments, standalone applications and systems.
- Method conversion/ coding: The process is time-consuming as the method needs coding, wherein the user requirements are provided by subject matter experts to coders, who don’t understand the workflow of the methods
- Risk assessment: Multiple tools are required to assess risk, as complex integration solutions have multiple applications and systems. It is important to assure compliance and sustainability of the solution and make it risk proof.
- Validation plan: Typically, in case of integrated electronic systems, this involves hardware as well as computer system validation, including various software applications that may require a detailed approach to deal with its complexity.
- Dealing with technology: The challenge is to find technology that can host all applications in one place for e.g. from ERP system to standalone laboratory instruments and applications.
- Training the user base: An enterprise solution requires proper training & management of a large number of skilled users, and system administrators.
Value creation and opportunity
Digital laboratories can be connected to and integrated with other laboratory informatics systems: laboratory information management systems (LIMS), analytical instrumentation, chromatography data systems (CDS), scientific data management systems (SDMS), modelling and simulation programs, chemical inventory systems and scientific databases, and business-level systems such as document management systems, systems applications products and manufacturing execution systems. The electronic lab environment provides a number of advantages to the industry.
Digital capabilities can create value across the five most common areas where lab metrics are measured.
- Innovation – Smart informatics systems reveal & highlight hidden data, and expose new insights that were previously impossible or unanticipated.
- Data Quality – Digital quality assurance/quality control (QA/QC) systems improve confidence in instrument data, interfaces, calculations and methodologies leading to a reduction in errors, improving product quality, enhancing Electronic Batch Record (EBR) support, and improving compliance and regulatory adherence. Digital systems also lead to a reduction in corrective and preventative action (CAPA) activities, fewer warning letters, and less audit fails.
- Data Security – Digitalization enables labs to prevent, report, manage and respond to data breaches with the right skills, tools, and processes.
- Operational Efficiency – Digital LIMS help accelerate laboratory test milestones. With this, organizations improve productivity and turnaround time, increasing customer satisfaction.
- Costs/Profitability – Digitalized labs lead to more efficient data exchange, use of laboratory resources, reagents, consumables, laboratory supplies and asset utilization.
Implementation success factors
Implementation of an integrated system(s) will typically involve consideration of many matters specific to integrated platforms, over current paper base fragmented laboratory systems. While developing a project plan/proposal, one should consider answering the following questions (indicative) with respect to cost, quality, ROI, project time, effort & resources required: –
- What could be the possible cost savings by implementing an Integrated Digital solution?
- What would be the quality increment?
- Which regulatory affairs the firm have to deal with?
- What kind of efficiency increment could the company reach?
- What is the effect of using an e-signature on current system?
- How far it will fill the request of data security?
- What is the cost of implementing a digital solution?
- What kinds of resources are required for life cycle management?
- Is it possible to interface to enterprise systems (i.e., enterprise resource planning or manufacturing execution system)?
- Which analytical instruments can be connected to the system?
- Which interface software package is required?
- What will be the influence on my company’s infrastructure?
- How big is the ROI?
Success can be defined by how well implementation minimizes expenses while maximizing savings and improving productivity.
To make implementation successful companies should consider three important factors: –
- Business case: In general, the business case for an integrated digital solution, can be harder to make. Business sponsors view digital laboratory deployment as an investment intended to improve analyst and reviewer efforts, typical laboratory process efficiency, people productivity and regulatory compliance. Thus, success is measured by ROI – the measure of money saved over a specified period of time.
- Expense control: Expenses in implementation include not just the cost of purchasing hardware and software but also configuring systems, instrument integration, training users, method coding, and maintaining systems over time (life cycle management).
- ROI: Success is measured by ROI, the measure of money saved over a specified period of time. W.r.t ROI, companies should consider various factors for e.g. project efficiency, reduction in cycle time, reduction in rework, time saved during information gathering process, productivity and efficiency increment and so on.
Conclusion and way forward
In an effort to reduce the cost of maintaining and supporting multiple fragmented digital solutions, organizations should consolidate onto one integrated digital solution, having integration with ERP, MES, LIMS, LES, CDS, SDMS and other enterprise and laboratory software applications – across all sites and geographies. It must be open, extensible, scalable, and robust. In addition, the digital solution must be easy to use, simplify the user’s work process, and be fast.
The roadmap to a digital laboratory does not simply end with adopting systems – it involves a cultural change -> how lab staff access, monitor and manage samples and results. They ultimately need to reskill and learn how to gather, record, view, retrieve and interpret data for true digital success in the lab.
 Author: VIPUL K. YAMDAGNI
Author: VIPUL K. YAMDAGNI
![]() Independent GMP Consultant and Adviser – This blog has been written with inputs from secondary sources .
Independent GMP Consultant and Adviser – This blog has been written with inputs from secondary sources .
Vipul Krishan Yamdagni is a seasoned Quality Assurance professional with a strong analytical frame of mind and a result-oriented approach.
He has spent over 34 years in the industry with major Indian pharma organizations like, Shriram Institute for Industrial Research, Morepen Laboratories, Dr. Reddy’s Laboratories, Ranbaxy Laboratories, Sun Pharmaceuticals and Glenmark Pharmaceuticals holding strategic leadership positions.
With diverse understanding and experience across departments like R&D, Supply Chain, Manufacturing, Project Management, Business Operations and Analytical Research he has spearheaded multiple ventures of setting state-of-art manufacturing and Quality systems and facilities, ensuring on time deliverables with GXP compliance.
Vipul is well known in the industry for his excellent technical knowledge, communication, interpersonal skills, team building and problem solving. Vipul has a proven track record of leading multiple teams and completing projects efficiently i.e. SAP, LIMS, Track-wise, Learning management system and Documentum as a function expert.
Vipul will be presenting at the first edition of the Paperless Lab Academy in India
Latest Posts
Press Release PLA® Conferences and 20/15 Visioneers forge strategic collaboration
Paperless Lab Academy® and 20/15 Visioneers forge strategic collaboration to shape the future of scientific and laboratory digital transformation. [M
07 February 2025
Meeting the Challenges of People, Process and Interfacing When Digitalising Labs
As the scientific and industrial landscape continues to evolve, digitalisation has become a cornerstone for innovation and efficiency in laboratory pr
20 January 2025
FAIR Principles and Data Stewardship Discussions at PLA2025Europe
FAIR Principles and Data Stewardship sustain any data centric approach Laboratories are striving for more and more automated and digitalised processes
12 December 2024

