From Raw Data to Artificial Intelligence

The Program of the next coming edition is getting pretty framed and we’re glad to share with you, that once again the presentations and discussion will cover the Data Life Cycle from Raw Data to Artificial Intelligence.
The Paperless Lab Academy® works in providing the audience food for thoughts in their digital transformation journey and in coordinating access to the latest technology offering of the lab informatics industry.
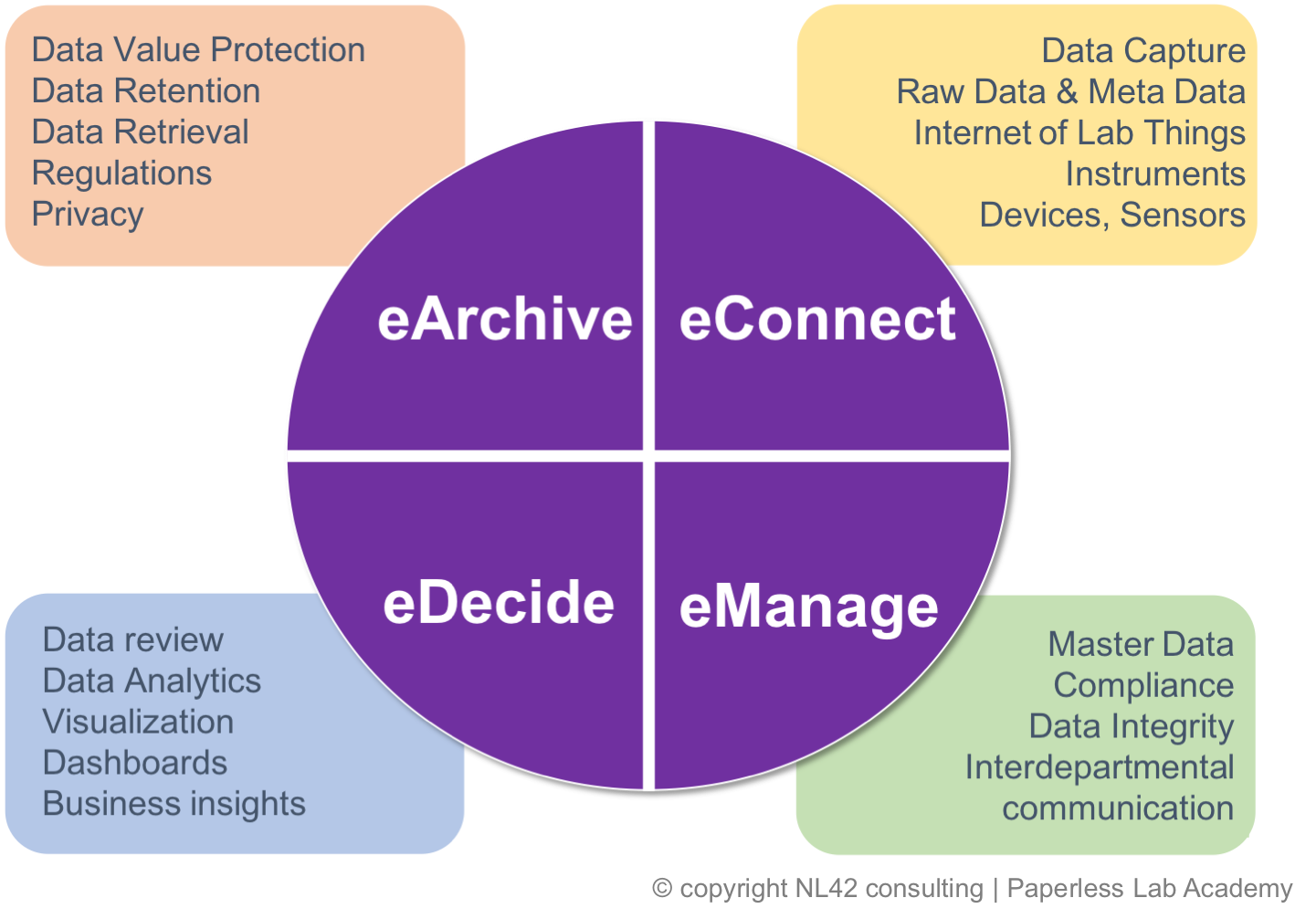 Fact is that when looking at laboratory and quality processes to be digitalised, several layers need consideration and multiple questions need answers. It is not always easy to even understand where to start and how to formulate decisions. A strong recommendation is to start with mastering your own data life cycle, and as such verify that the required regulatory compliance are observed in each steps and phases: eConnect, eManage, EDecide & eArchive.
Fact is that when looking at laboratory and quality processes to be digitalised, several layers need consideration and multiple questions need answers. It is not always easy to even understand where to start and how to formulate decisions. A strong recommendation is to start with mastering your own data life cycle, and as such verify that the required regulatory compliance are observed in each steps and phases: eConnect, eManage, EDecide & eArchive.
Master you Data Life Cycle
That is why we’ve collected a series of presentations developing further more from Raw Data capture moment to efficient use of the data for analytics, business and artificial intelligence.
Whether they are raw data from chromatographs or any other analytical instruments, they have been at the heart of many data falsification and poor data management practices found in many FDA warning letters since the Able Laboratories fraud case in 2005.
 Breaches to data integrity can be avoided by creating flawless data transfer from instruments to systems, including avoiding use of spreadsheets for calculations. If you’re open of being challenged We Mr. Bob McDowall, we warmly recommend you to attend his provocative yet funny and to the point presentation: Are Spreadsheets a Fast Track to Regulatory Non-Compliance?
Breaches to data integrity can be avoided by creating flawless data transfer from instruments to systems, including avoiding use of spreadsheets for calculations. If you’re open of being challenged We Mr. Bob McDowall, we warmly recommend you to attend his provocative yet funny and to the point presentation: Are Spreadsheets a Fast Track to Regulatory Non-Compliance?
Consider an electronic process with validated calculations and electronic signatures
 The message will be reinforced by Mr. Mark Newton´s presentation on Data Forensics with a pragmatic example and exercise about the importance of metadata. The information about the data that brings a deeper understanding about the quality of the final results.
The message will be reinforced by Mr. Mark Newton´s presentation on Data Forensics with a pragmatic example and exercise about the importance of metadata. The information about the data that brings a deeper understanding about the quality of the final results.
Mark is a co-leader for the GAMP Data Integrity Special Interest Group and Chair of ISPE Global Documents Committee. Co-author of “Harmonizing USP <1058> and GAMP for Analytical Instrument Qualification” and Co-editor of the GAMP Good Practice Guide “A Risk-Based Approach to Compliant Computerized Laboratory Systems”.
Systems Integration approaches
 Yet more instruments are involved in a analytical processes and additional metadata and unstructured data are to be facilitated alongside the raw data. Andreas Steinle from Roche Diagnostic will take us through their very specific Instrument integration strategy and journey and explain how to unlock your analytical instrument data for data science
Yet more instruments are involved in a analytical processes and additional metadata and unstructured data are to be facilitated alongside the raw data. Andreas Steinle from Roche Diagnostic will take us through their very specific Instrument integration strategy and journey and explain how to unlock your analytical instrument data for data science
 Mr. Vipul Krishan Yamdagni, now independent GMP consultant and advisor, brings 34 years experience in the industry with major Indian Pharma organisations about digital strategy and systems integration. Vipul will share his view on Digital Pharma Laboratory – challenge | opportunity | way forward
Mr. Vipul Krishan Yamdagni, now independent GMP consultant and advisor, brings 34 years experience in the industry with major Indian Pharma organisations about digital strategy and systems integration. Vipul will share his view on Digital Pharma Laboratory – challenge | opportunity | way forward
Implementation experiences by time of COVID
Despite the pandemic situation and the lockdown, manufacturing companies had to react quickly to the situation, remain productive while implementing safety procedures for their employees. Three presenters will explain us how they’ve overcome the situation, implemented solutions and taken benefit of them during this though and challenging moment.
 Ms. Preeti Gaonkar, SEAP Quality Excellence Manager at SGS India Private Limited will explain how they have accelerated their quality with digital transformation. By implementing of a Quality Management System digital system they could incorporate efficient electronic workflows to manage their quality events, audits, SOPs, quality documents and employee training records.
Ms. Preeti Gaonkar, SEAP Quality Excellence Manager at SGS India Private Limited will explain how they have accelerated their quality with digital transformation. By implementing of a Quality Management System digital system they could incorporate efficient electronic workflows to manage their quality events, audits, SOPs, quality documents and employee training records.
 Mr. Albert Almajano, Chief Information Officer at Indukern Group will share their company strategy and experience in remaining focused to the implementations during the lockout situation with his presentation, Albert will show us a Roadmap to Digitalisation with Success
Mr. Albert Almajano, Chief Information Officer at Indukern Group will share their company strategy and experience in remaining focused to the implementations during the lockout situation with his presentation, Albert will show us a Roadmap to Digitalisation with Success
 Mr. Sergio Nasi, Head of IT Operations Laboratory Execution at Boehringer-Ingelheim will provide and overview of an ongoing implementation of a laboratory execution system and explain how additional challenges and opportunities the global COVID-19 pandemic have meant to the project implementation. Sergio will help us to Define your own pathway to the paperless QC laboratory: lessons learned from an ongoing project
Mr. Sergio Nasi, Head of IT Operations Laboratory Execution at Boehringer-Ingelheim will provide and overview of an ongoing implementation of a laboratory execution system and explain how additional challenges and opportunities the global COVID-19 pandemic have meant to the project implementation. Sergio will help us to Define your own pathway to the paperless QC laboratory: lessons learned from an ongoing project
Data Analytics and Artificial Intelligence
 Toni Manzano, Chief Science Officer, R&D Director and Co-founder at Bigfinite brings a strong experience on data analytics, machine learning, artificial intelligence and sound knowledge on all related algorithms and statistics concepts behind. A unique opportunity to listen to an AI specialist with strong domain knowledge of the manufacturing industries.
Toni Manzano, Chief Science Officer, R&D Director and Co-founder at Bigfinite brings a strong experience on data analytics, machine learning, artificial intelligence and sound knowledge on all related algorithms and statistics concepts behind. A unique opportunity to listen to an AI specialist with strong domain knowledge of the manufacturing industries.
Do not miss the opportunity to benefit from his presentation and learn about Data Centricity: What is behind the AI implementation in Pharma
 Dr. Lloyd F. Colegrove, Director at Dow Chemicals, now retired and consultant, has spent 7 years in Polymer Research in Dow (R&D and TS&D) before moving into Manufacturing in an Analytical Improvement role and then a Quality Leader for five Dow businesses before moving to help establish a new capability called Fundamental Problem Solving, where top chemists and engineers work on complex, often multi-effect, plant problems. With this established, he refocused his efforts working to establish the data initiatives that support this work. Lloyd´s talk “Digital Transformation in the Chemical Industry. The Devil’s in the Details” will feature artificial intelligence as an example of a key future value delivery mechanism in digital transformation. The road to installing AI in the Chemical Industry is fraught with challenges and many dead ends.
Dr. Lloyd F. Colegrove, Director at Dow Chemicals, now retired and consultant, has spent 7 years in Polymer Research in Dow (R&D and TS&D) before moving into Manufacturing in an Analytical Improvement role and then a Quality Leader for five Dow businesses before moving to help establish a new capability called Fundamental Problem Solving, where top chemists and engineers work on complex, often multi-effect, plant problems. With this established, he refocused his efforts working to establish the data initiatives that support this work. Lloyd´s talk “Digital Transformation in the Chemical Industry. The Devil’s in the Details” will feature artificial intelligence as an example of a key future value delivery mechanism in digital transformation. The road to installing AI in the Chemical Industry is fraught with challenges and many dead ends.
Those are the topics and presenters that you should expect to listen to and discuss about during the Paperless Lab Academy 2020 India online event.
Make sure to Register for you to access the virtual event platform !
Latest Posts
Press Release: PLA® Conferences to partner with IA-Meetings for its 5th Indian Edition.
The Paperless Lab Academy® (PLA) is a leading conference about digital transformation of laboratory and quality processes. Above all, it is about mas
08 April 2024
The #PLA2024Europe programme aimed to highlight the importance of the human factor in digital transformation with several presentations and panel disc
19 March 2024
PLA2024Europe Workshops at your fingertips
The PLA® Conference workshops are synonymous with interactive sessions and discussions with subject matter experts. Most importantly, these workshops
13 February 2024

